40 chemistry potential energy diagram
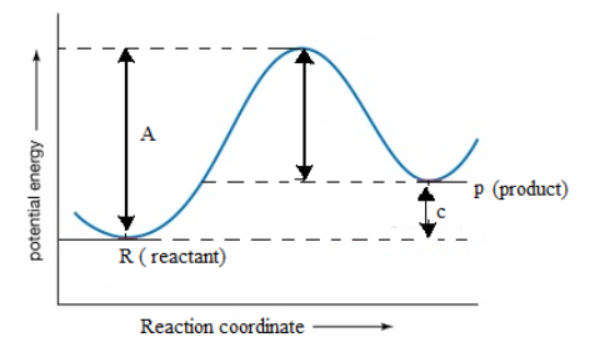
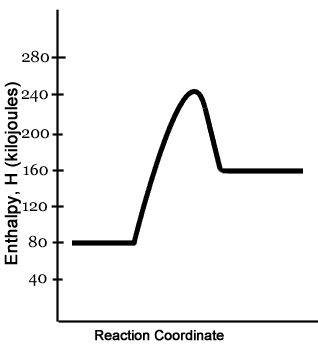
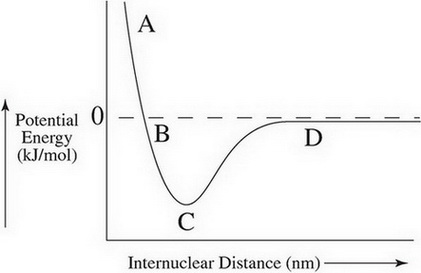
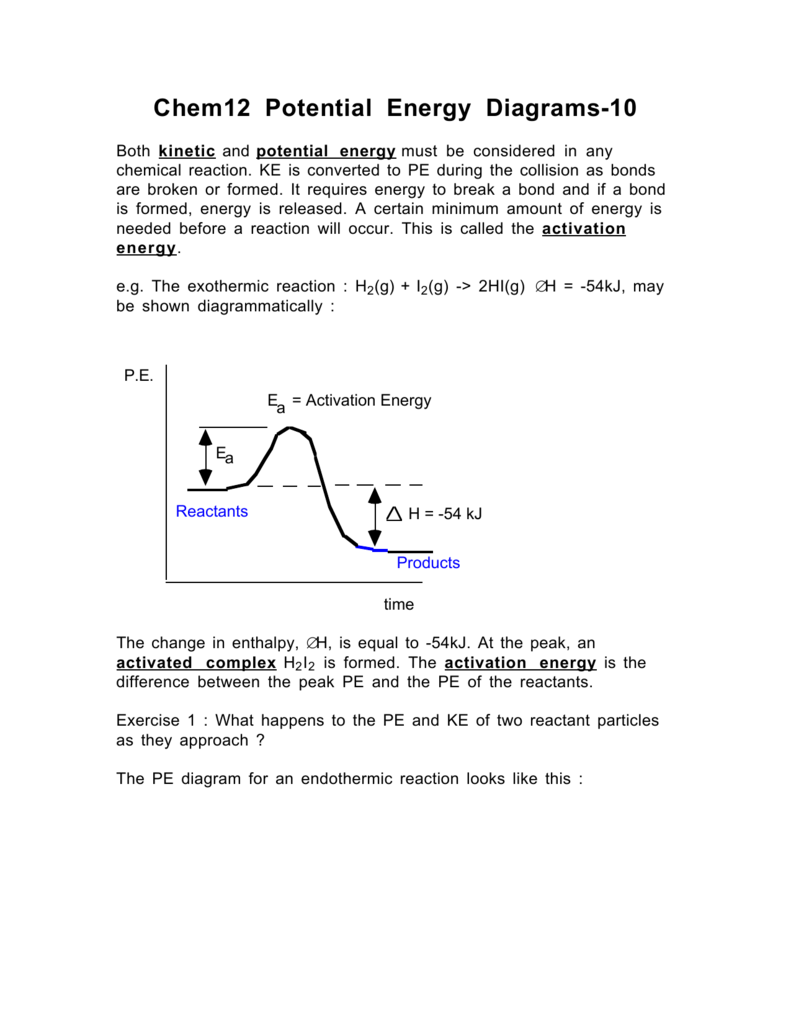
Potential energy diagrams Consider an arbitrary potential energy shown schematically below. There are a number of important qualitative features of the behavior of the system that can be determined by just knowing this curve. The first thing to notice is that since the kinetic energy The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram, or sometimes called a reaction progress curve. A potential energy diagram shows the change in potential energy of a system as reactants are converted into products.
A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic ...Question: Answer

Chemistry potential energy diagram
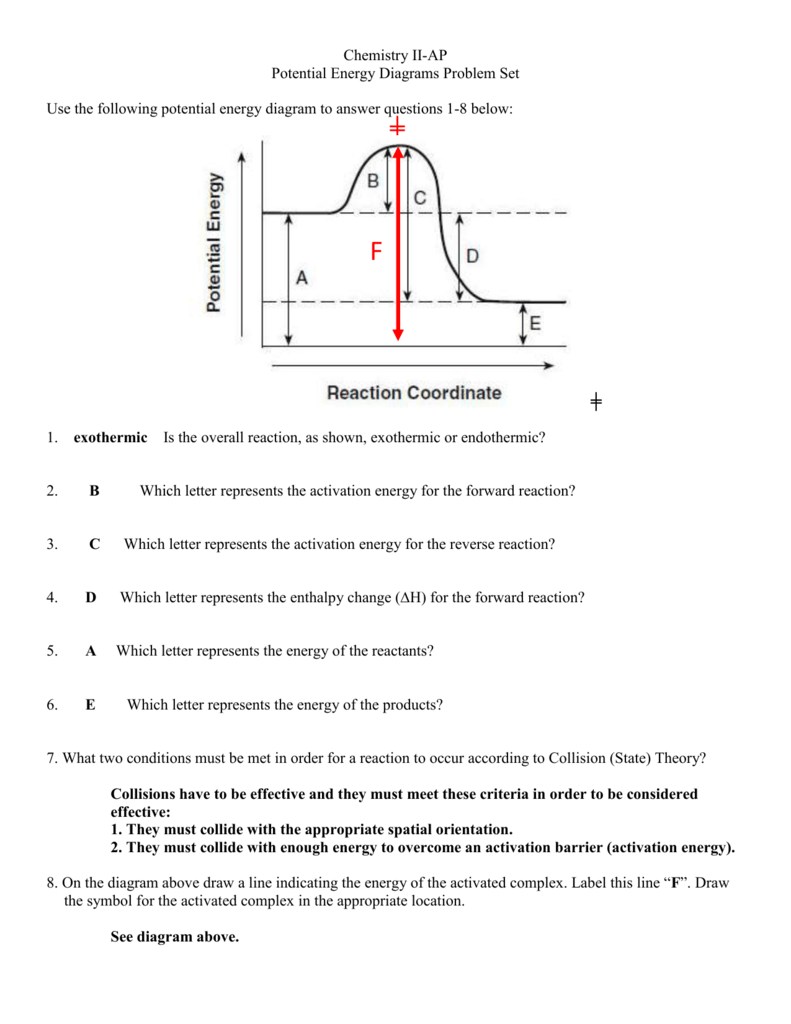
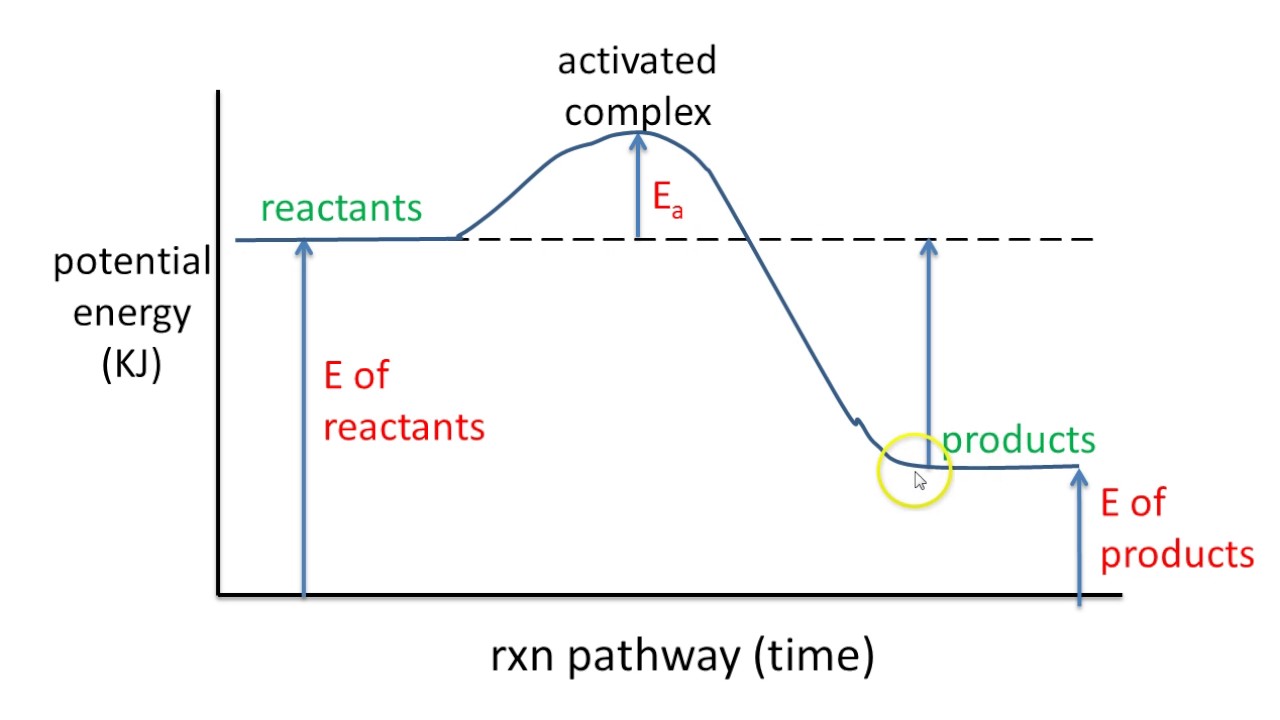
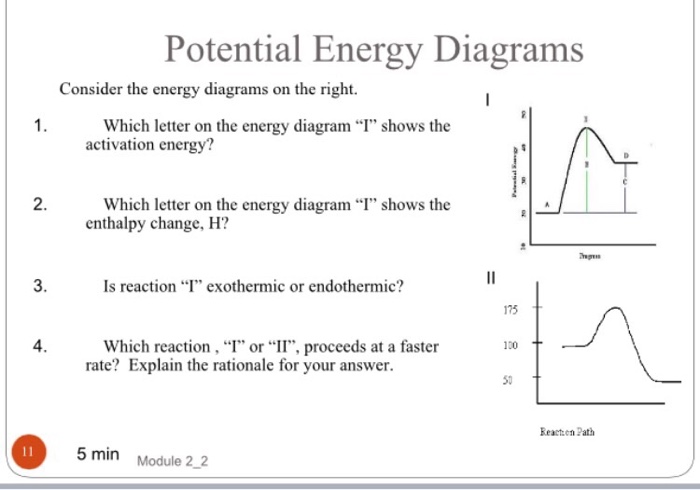
9 Jul 2021 — The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram, or sometimes called a ... Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what determines the rate at which the reaction occurs? The reaction whose potential energy diagram is shown in the figure is a two-step reaction. The activation energy for each step is labeled E a1 and E a2 .Each elementary step has its own activated complex, labeled AC 1 and AC 2 .Note that the overall enthalpy change of the reaction is unaffected by the individual steps, since it depends only on the initial and final states.
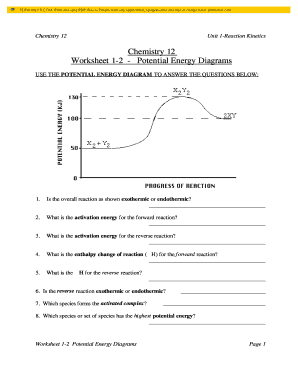
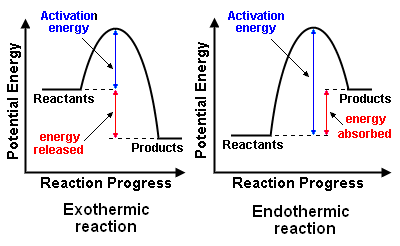
Chemistry potential energy diagram. 9 Jul 2019 — The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram, or sometimes called a ... Explains potential energy diagrams and activation energy. We have moved all content for this concept to for better organization. Please update your bookmarks accordingly. 1 | Potential Energy Diagrams Worksheet Potential Energy Diagrams Worksheet CK-12 Foundation Chemistry Name Use the following Potential Energy Diagram to answer questions 1 - 12. 150 100 Potential Energy X2+Y2 50 Progress of Reaction 1. Is the overall reaction as shown exothermic or endothermic? 2. 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
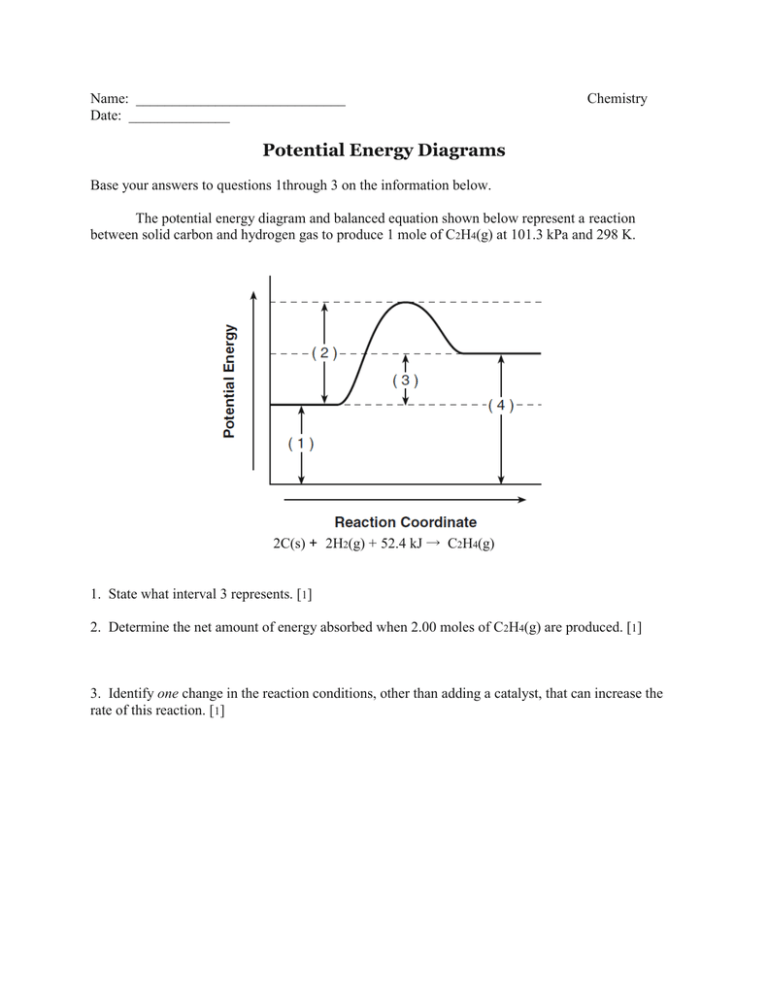
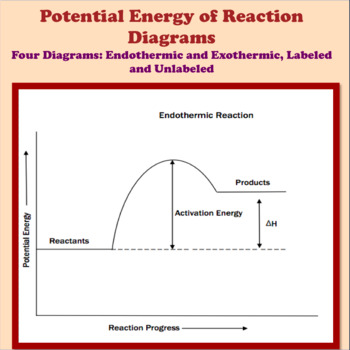
Part I: Potential Energy Diagrams Read pages 527-529 in Chemistry: the Central Science. The energy changes involved in chemical reactions can be conveniently examined using potential energy diagrams. The total potential energy of all the reactants in the reaction is illustrated at the left of the diagram, the total potential energy of the ... 23 Feb 2012 — Potential energy diagrams illustrate the potential energy of the reactants and products for a chemical reaction and how this energy changes ... A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the energy of the products is greater than the energy of the reactants and ΔH is positive. (B) In an exothermic reaction, the energy of the products is lower than the energy of the reactants and ΔH is ... Potential energy diagrams. Chemical reactions involve a change in energy, usually a loss or gain of heat energy. The heat stored by a substance is called its enthalpy (H). \ (\Delta H\) is the ...
The reaction whose potential energy diagram is shown in the figure is a two-step reaction. The activation energy for each step is labeled E a1 and E a2 .Each elementary step has its own activated complex, labeled AC 1 and AC 2 .Note that the overall enthalpy change of the reaction is unaffected by the individual steps, since it depends only on the initial and final states. Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what determines the rate at which the reaction occurs? 9 Jul 2021 — The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram, or sometimes called a ...



















0 Response to "40 chemistry potential energy diagram"
Post a Comment