40 molecular orbital diagram of benzene
The Pi Molecular Orbitals of Benzene - Master Organic ... The number of pi molecular orbitals in the pi-system equals the number of contributing atomic p orbitals. For butadiene (n=4) we saw that the energy levels of the pi system stacked like a four-story apartment building. Both hexatriene and benzene have six contributing p-orbitals (n = 6), so we should expect six pi orbitals for each. Role of molecular orbitals of the benzene in electronic ... In an effort to examine the intricacies of electronic nanodevices, we present an atomistic description of the electronic transport properties of an isolated benzene molecule. We have carried out ab initio calculations to understand the modulation of the molecular orbitals (MOs) and their energy spec …
Molecular Orbitals of Benzene - YouTube Donate here: video link: link: ...

Molecular orbital diagram of benzene
Molecular orbital structure of Benzene || History of ... structure of benzene,molecular orbital picture of benzene,molecular orbital structure of benzene,benzene,pi molecular orbitals of benzene,orbital structure o... Benzene Lewis Structure, Molecular Geometry, Hybridization ... Benzene Molecular Orbital (MO) Diagram A molecular orbital (MO) diagram explains the chemical bonding in molecules by energy level diagrams. They were proposed by Robert S. Mulliken and Friedrich Hund in 1928. The postulates of MO theory are as follows- Electrons present in molecular orbitals of a molecule are considered. π-Molecular Orbitals of Benzene π-Molecular Orbitals of Benzene. How to Manipulate JSmol Structures or Click on the JSmol logo. There may be a slight delay in the loading of orbitals. Select a π-Molecular Orbital Commands . The diagram below is a Frost-Hückel circle mnemonic. A polygon representing the ring system, in this case a regular hexagon for benzene, is inscribed ...
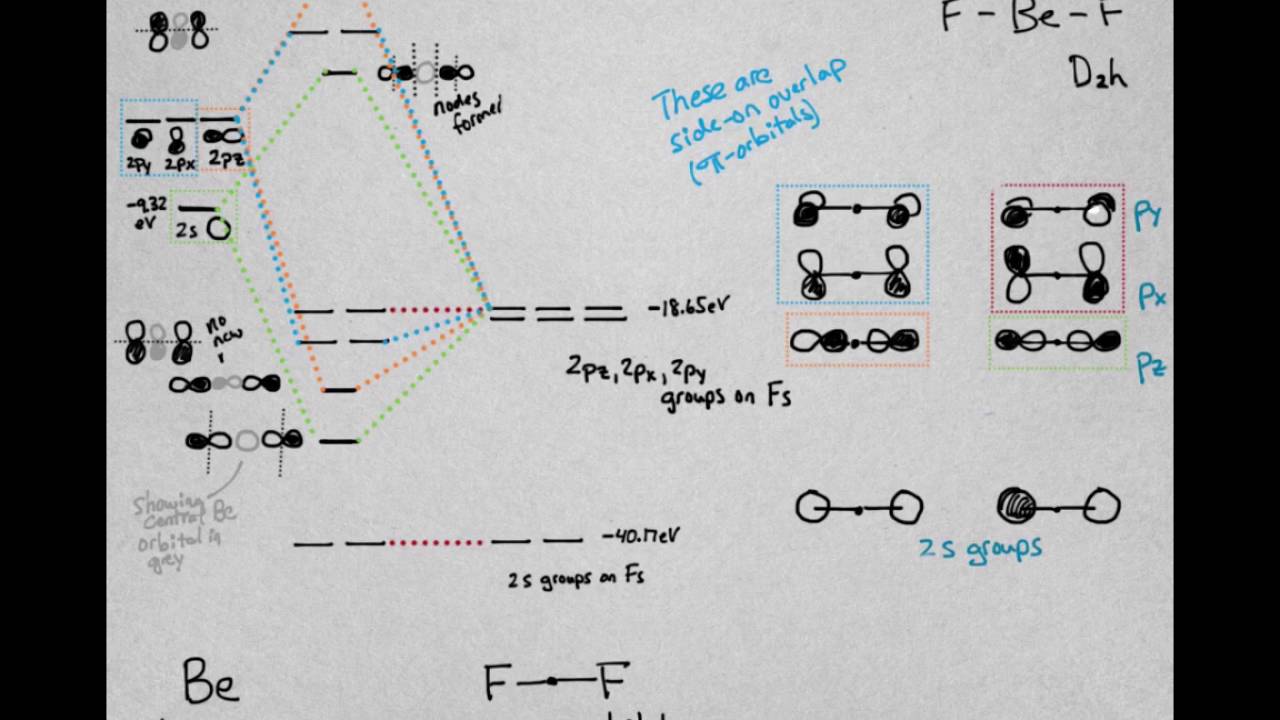
Molecular orbital diagram of benzene. Explain the molecular orbital structure of benzene class ... Benzene is an organic compound that has the formula of C 6 H 6. It is an aromatic compound and consists of a single ring structure . The carbon atoms are s p 2 hybridised in benzene and all of them lie in the same plane. They have a 120 0 angle orientation. PDF Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals. Discuss the molecular orbital structure of benzene ... The orbital structure of benzene: All the carbon atoms in benzene are sp2 hybridised. The three sp2 hybrid orbitals are lying in one plane and oriented at an angle of 120°. The fourth unhybridized p-orbital having two lobes is lying perpendicular to the plane of the hybrid orbital. Two out of the three sp2 hybrid orbitals of each carbon atom overlap axially with sp2 hybrid orbitals of the ... Explain the molecular orbital structure of benzene class ... Since 2 p z orbital on any carbon atom can overlap sideways with the 2 p z orbital on adjacent carbon atom on either side equally well, a continuous T-molecular 3 orbitals will result which embraces all the six p-electrons as shown: In benzene molecules all C-C-C and H-C-C are of 120 ∘ each and each C-C bond length is 139pm.
15.3: Pi Molecular Orbitals of Benzene - Chemistry LibreTexts An electrostatic potential map of benzene, shown below, shows that the pi electrons are evenly distributed around the ring and that every carbon equivalent. Each carbon in the benzene ring is sp2 hybrized with a p orbital perpendicular to the ring plane (Left) Being planar and cyclic allows benzene's p orbitals to undergo cyclic overlap (Right) Aromaticity - π Molecular Orbitals of Benzene Aromaticity - π Molecular Orbitals of Benzene CONTROLS Click the orbital levels to view the molecular orbitals The calculated energy of the molecular orbital is shown in the left corner. • Observe the degeneracy of pairs of molecular orbitals. • Increasing number of nodes corresponds to increasing energy. 159 4.5 ( 13) How useful was this page? PDF Lecture Notes | Physical Chemistry - MIT OpenCourseWare Molecular orbital theory, H 2 L27 LCAO-MO theory L28 Qualitative molecular orbital theory L29 Modern electronic structure theory L30 Interaction of light with matter L31 Vibrational spectra L32 NMR spectroscopy I L33 NMR spectroscopy II L34 Perturbation theory L35 Vibrational anharmonicity L36 Solved Below are the pi molecular orbitals of benzene ... Transcribed image text: Below are the pi molecular orbitals of benzene. Rank them in order of increasing energy based on the number of nodes. Consider that the blue color indicates one phase and the red color indicates the other phase of the orbital. Rank from lowest energy to highest energy. To rank items as equivalent, overlap them.
Projection operator method: pi molecular orbitals of ... Derivation of the pi molecular orbitals of benzene (C₆H₆), using the projection operator method.00:09 Reducible representation for pi group orbitals08:01 Red... Explain the molecular orbital structure of benzene? Explain the molecular orbital structure of benzene? >>. Class 11. >> Chemistry. >> Chemical Bonding and Molecular Structure. >> Molecular Orbital Theory. Six π- Molecular Orbitals (MO's) of Benzene Six π- Molecular Orbitals (MO's) of Benzene. How to Manipulate JSmol Structures or Click on the JSmol logo. The diagram below is a Frost-Hückel circle mnemonic. A polygon representing the ring system, in this case a regular hexagon for benzene, is inscribed in a circle with a vertex at the bottom. The energy levels of the six-π-MO's are ... Solved the following is pi molecular orbitals of benzene ... In the orbital diagram, show which molecular orbitals are filled in benzene's ground state. (Place the electrons in the correct molecular orbital) Show transcribed image text Expert Answer 100% (1 rating) Benzene has 3 π bonds and hence a total of 6 π electrons. Please note that the … View the full answer Transcribed image text: 30.
Molecular Orbitals - College of Saint Benedict and Saint ... Note that, like in benzene, when there are multiple ways to represent a combination, one molecular orbital is usually represented to show both bonding and antibonding interactions. The other usually shows the net result, either just bonding or antibonding or, in the case of cyclobutadiene, no direct interaction between adjacent atoms because it ...
The electrons in the delocalized π molecular orbitals of ... The electrons in the delocalized π molecular orbitals of benzene (C6H6) asked Aug 23, 2019 in Chemistry by MostSecret. A. are free to move around the six-membered ring. B. give rise to alternating single and double bonds around the ring. C. form the electron pairs in the C-H bonds of the compound. D. are unevenly distributed through the molecule.
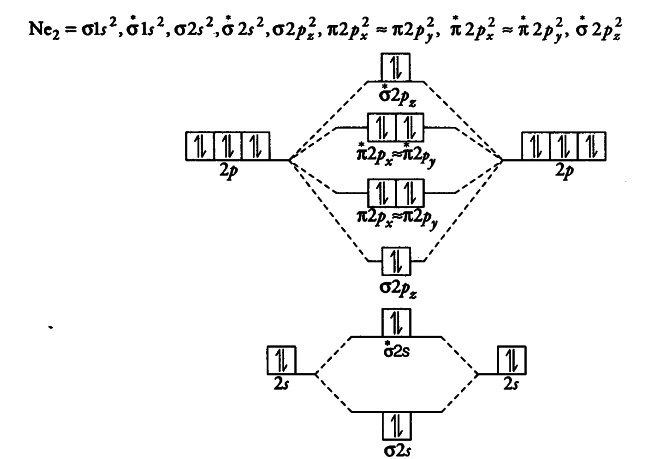
Molecular orbitals - SlideShare Molecular Orbital Diagram This is a molecular orbital energy level diagram for the p orbitals. Note that the σ bonding orbital is lowest in energy due to the greater overlap end-onend. σu πg 2p πu σg 2p 29. Molecular Orbital Diagram The alternate notation is provided on the right side of the energy level diagram. σu πg 2p πu σg 2p 30.
Molecular Orbital Treatment of Benzene-bond Lingth ... These 2pz-orbital are perpendicular to the plane of sigma bonds. Pz orbital in benzene These 2pz-orbitals by lateral o verlapping form three alternate pi-bonds in benzene ring.There are two possibilities of pi-bond formation in benzene. OR Actually these 2pz-orbital produce a pi-molecular orbital containing six electrons.
The Pi Molecular Orbitals of Benzene - Purdue University The Pi Molecular Orbitals of Benzene (D 6h Symmetry) Note: due to the size of the orbital files, it may take several seconds for the orbitals to appear. Note that only the total electron density is shown for each orbital (i.e., the phases for each orbital are not shown). 6 (b 2g) 4 (e 2u)
How many molecular orbitals Does benzene have? Quantum mechanical calculations tell us that the six pi molecular orbitals in benzene, formed from six atomic p orbitals, occupy four separate energy levels. pi 1 and pi 6 * have unique energy levels, while the pi 2 - pi 3 and pi 4 *- pi 5 * pairs are degenerate, meaning they are at the same energy level.
Pi Molecular Orbitals 1,3 Butadiene - Chad's Prep® ψ1 is a bonding molecular orbital, is occupied in the ground state, and is the Highest Occupied Molecular Orbital (HOMO). ψ2* is an antibonding molecular orbital, is unoccupied in the ground state, and is the Lowest Unoccupied Molecular Orbital (LUMO). The orbitals are arranged in the following table in order of increasing energy.




0 Response to "40 molecular orbital diagram of benzene"
Post a Comment