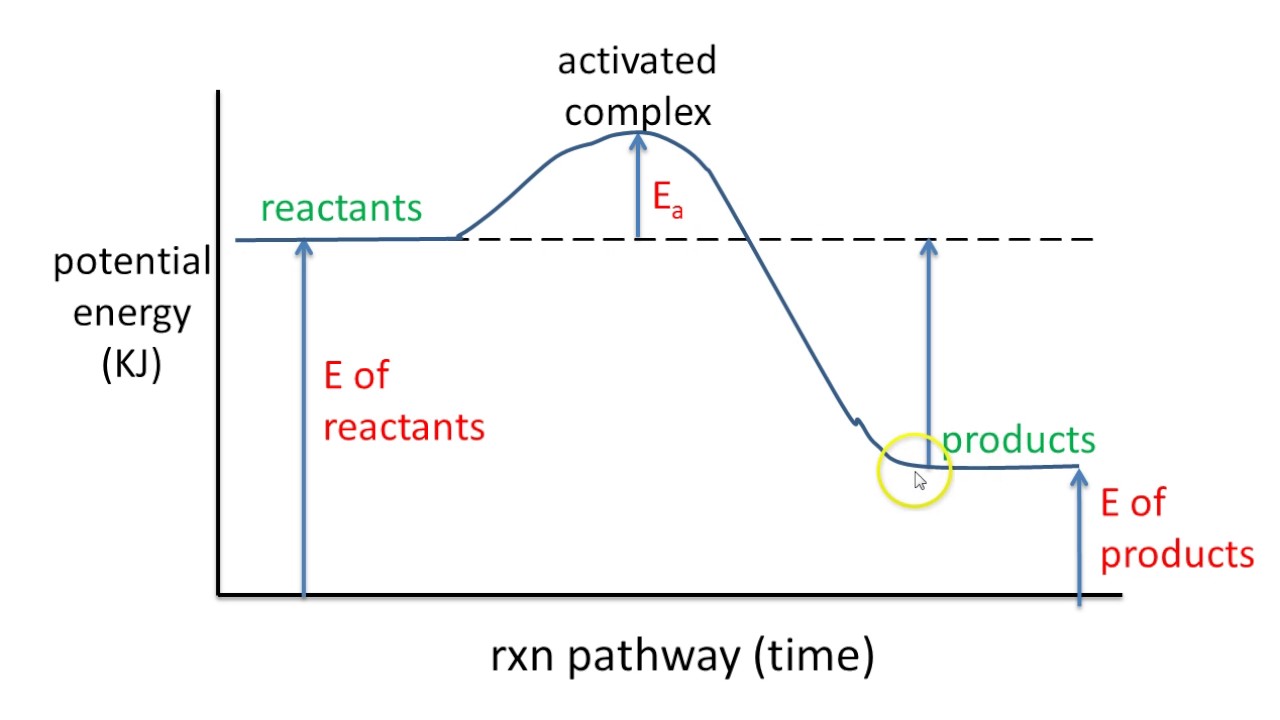
40 potential energy diagram endothermic
Solved Select the potential energy diagram that represents ... Select the potential energy diagram that represents a two-step endothermic (endergonic) reaction. I II III IV; Question: Select the potential energy diagram that represents a two-step endothermic (endergonic) reaction. I II III IV. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text EXOTHERMIC & ENDOTHERMIC REACTIONS: ENERGY ... ENERGY DIAGRAM. • A = POTENTIAL ENERGY OF REACTANTS. • B = ACTIVATION ENERGY. • ENERGY REQUIRED TO START THE REACTION. • C = ACTIVATION ENERGY (REVERSE.11 pages
Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction. In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. ... In endothermic reactions, there is less energy in the reactants than ...

Potential energy diagram endothermic
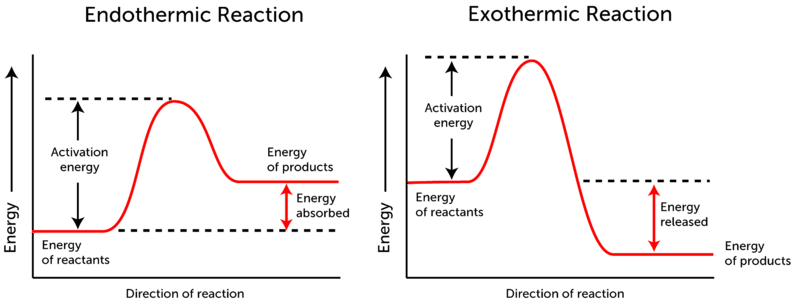
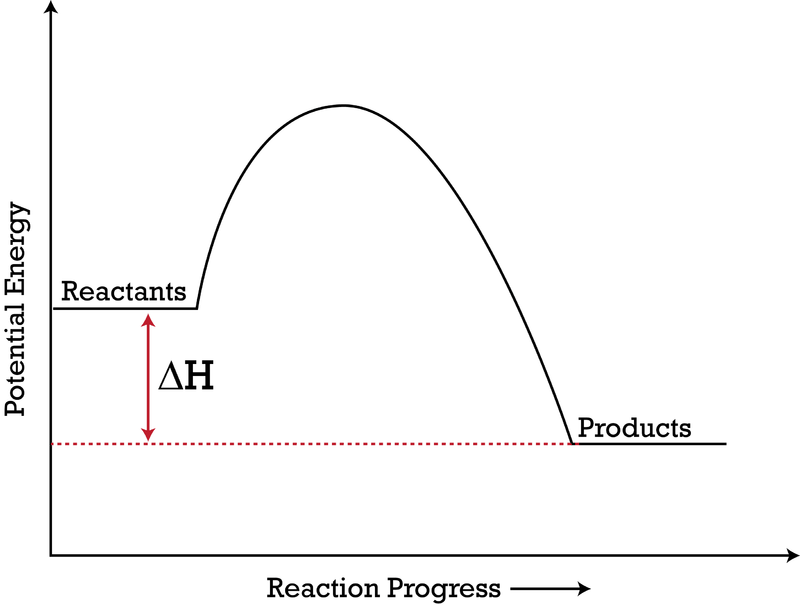
What are Endothermic Reactions? (with Examples & Video) The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant. Endothermic and Exothermic Reactions With Potential Energy ... This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams.... potential_energy_diagrams_worksheet (1).docx - REACTION ... On the potential energy diagram indicate where the activated complex will be formed. Enthalpy Change As shown above, when reactions occur there will be a difference in potential energy between the reactants and products. This difference in potential energy is known as the enthalpy change and is given the symbol ΔH. The units for ΔH are kJ mol -1 .
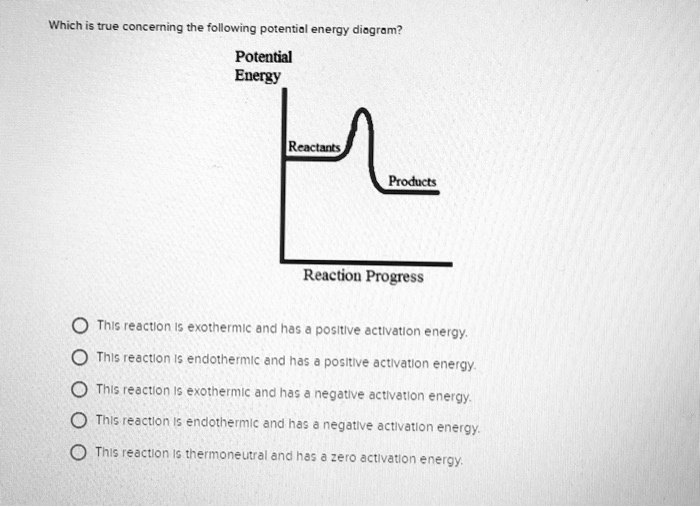
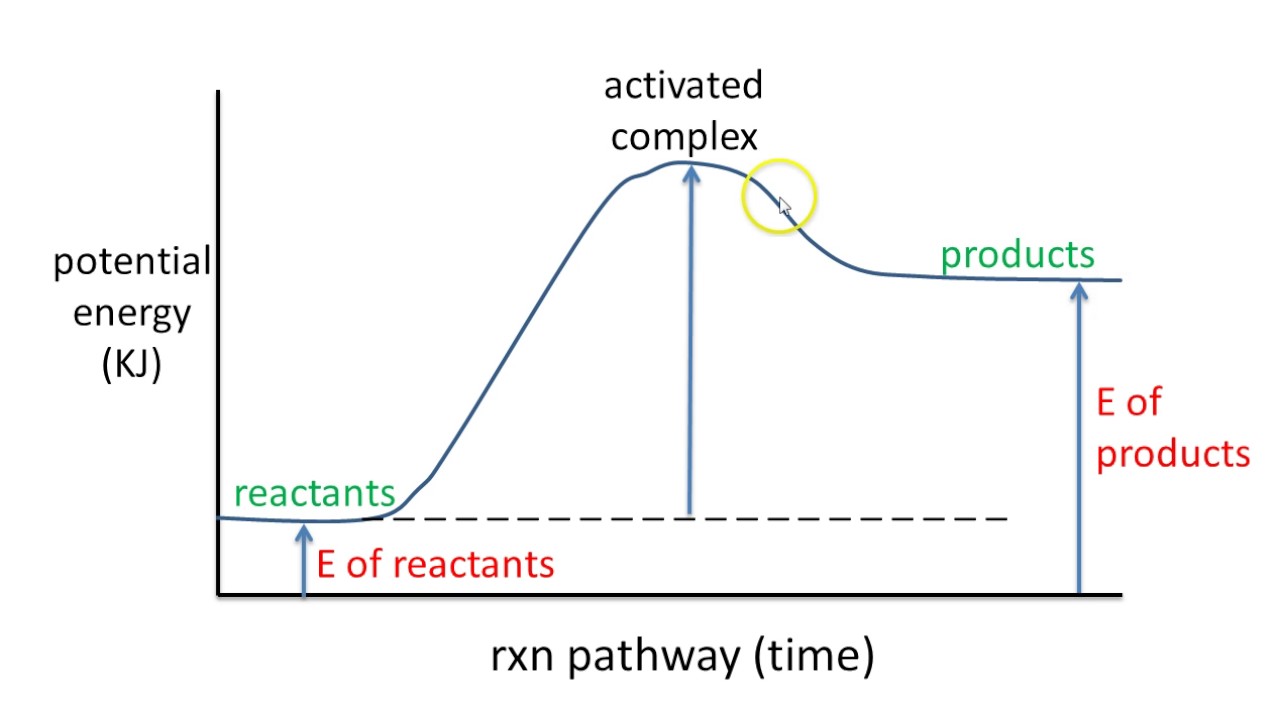
Potential energy diagram endothermic. 3.15: Exothermic and Endothermic Processes - Chemistry ... Figure 3.15. 3: A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the energy of the products is greater than the energy of the reactants and ΔH is positive. PDF Potential Energy Diagrams POTENTIAL ENERGY DIAGRAMS PURPOSE POTENTIAL ENERGY DIAGRAMS ARE A VISUAL REPRESENTATION OF POTENTIAL ENERGY IN A CHEMICAL REACTION NOTE THE X AXIS IS USUALLY REACTION CORDINATE OR TIME NOTE THE Y AXIS IS POTENTIAL ENERGY WHERE IN THE REACTION PROGRESS/TIME IS THE POTENTIAL ENERGY HIGHEST? PURPOSE CONT. Which statement describes the potential energy diagram of ... Answer: option C. The potential energy of the products is greater than the potential energy of the reactants. Explanation: 1) The potential energy diagram shows the evolution of the potential chemical energy of a process or reaction, measured as energy of the chemical bonds, including the reactants, the intermediates, the activated complex, and the products. Potential Energy Diagrams | Chemistry for Non-Majors A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction.
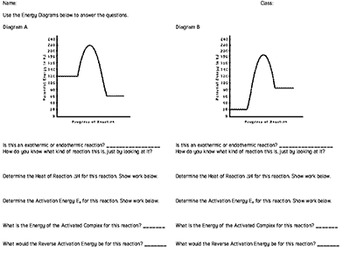
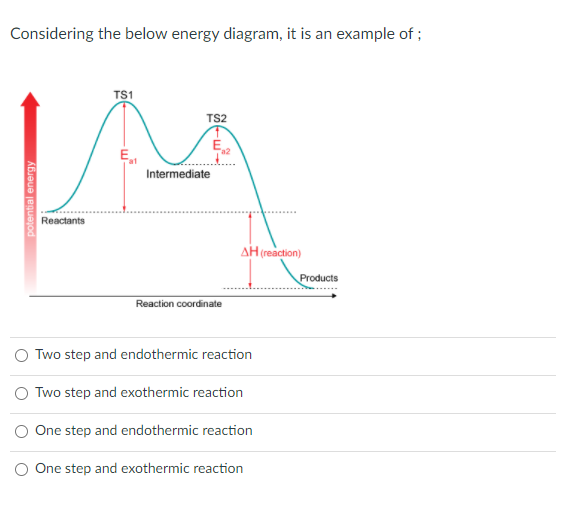
Endothermic Potential Energy Diagram - Summarized by Plex ... The figure below illustrates basic potential energy diagrams for an endothermic and an exothermic reaction. As the system absorbs electricity from the environment, the total potential energy of the system rises for the endothermic reaction. For this reason, the activation energy of a reaction is often referred to as the activation energy barrier. Potential Energy Diagrams - Kentchemistry.com According to Table I, which potential energy diagram best represents the reaction that forms H2O(l) from its elements? Answer--> Choice 1, since H is negative 3. Given the reaction: CH4(g) + 2 O2(g) --> 2 H2O(l) + CO2(g) What is the overall result when CH4(g) burns according to this reaction? (1) Energy is absorbed and ΔH is negative. Potential Energy Diagram Practice Endothermic and ... Students will be given two Potential Energy Diagrams -- one Endothermic, one Exothermic. They will have to read the graph and determine if the graph is showing an endothermic or exothermic reaction, justify why it is so, then calculate the heat of reaction (delta H), activation energy (Ea), reverse activation energy, answer four conceptual multiple choice questions, and then determine if 9 ... Endothermic Reaction Energy Diagram - 30 energy diagram ... Endothermic Reaction Energy Diagram - 14 images - dr will mccarthy s science site endothermic vs exothermic, energy profiles chemistry tutorial, exothermic and endothermic changes, exergonic reaction gibbs free energy chemical reaction,
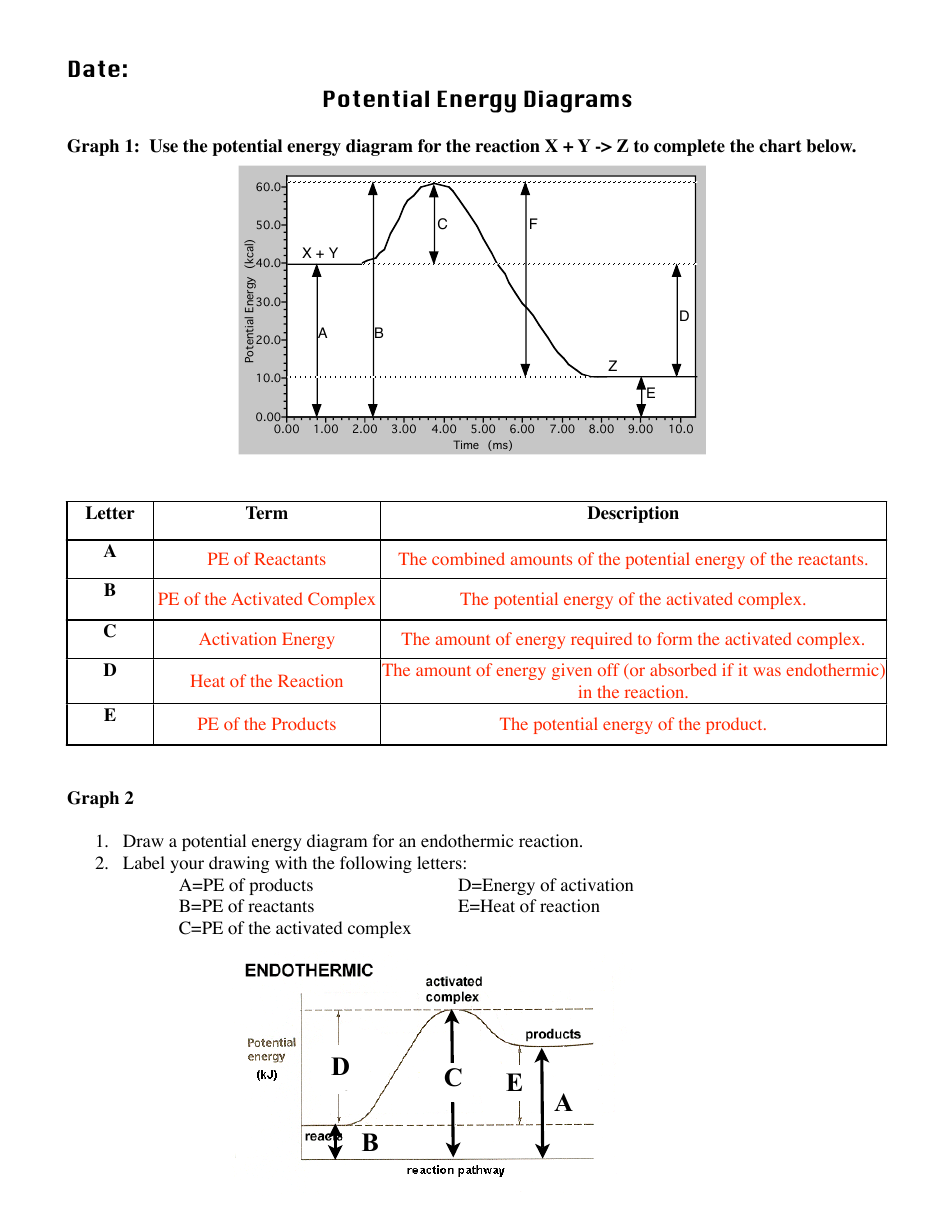
How can I represent an endothermic reaction in a potential ... Dec 20, 2015 — Why is the respiration reaction exothermic? Why is combustion an exothermic reaction? How can I read the potential energy diagrams when there is ...1 answer · An endothermic reaction must have a positive change in enthalpy. That is, ΔH>0. This means that the system absorbs heat. ... 18.4: Potential Energy Diagrams - Chemistry LibreTexts The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change \ (\left ( \Delta H \right)\) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the potential energy diagrams. Which statement describes the potential energy diagram of ... 04/29/2019 Chemistry High School answered • expert verified Which statement describes the potential energy diagram of an endothermic reaction? A. The potential energy of the products is equal to the potential energy of the reactants. B. The potential energy of the reactants is greater than the potential energy of the products. C. Endothermic and Exothermic Activity.pdf - Endothermic and ... Endothermic and Exothermic Activity For this assignment, you will create your own potential energy diagrams for each of the three chemical reactions. Then you will analyze the data and your diagrams for each reaction. Generic Reactions Reactants Products Transition State Synthesis A + B → AB A + B −15 kJ AB 20 kJ 30 kJ Single Replacement C + AB → CB + A C + AB 65 kJ CB + A 30 kJ 85 kJ ...
PPT Energy Diagrams: Drawing it Out energy diagrams display the energy of reactants and products the energy needed for a reaction to occur the energy absorbed or released due to reaction energy diagram parts potential energy of the reactants potential energy of the products activation energy e - the amount of energy required to reach the transition state or activated complex from …
Representing endothermic and exothermic processes using ... A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state. If the initial state has a lower potential energy than the final state, the process is endothermic.
PDF Potential Energy Diagram Worksheet ANSWERS The reverse reaction is ____exothermic_____ (endothermic or exothermic). Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what ...
Chemistry 7.01 Live Lesson, very important - Mrs. Evans 7 ... Endothermic and Exothermic Activity. For this assignment, you will create your own potential energy diagrams for each of the three chemical reactions. Then you will analyze the data and your diagrams for each reaction. Generic Reactions. Reactants Products Transition State. Synthesis. A + B → AB. A + B −15 kJ AB 20 kJ 30 kJ. Single Replacement
Solved Endothermic reactions 5. In the space below, draw a ... Catalysis 8. The following diagram is the potential energy diagram for the same exothermic reaction as in question 1. produsenere Supe Stage Reaction coordinate On the diagram, draw a dotted line to show the pathway of a catalyzed reaction. Add the label C to show the interval that represents the activation energy for the catalyzed reaction. 9.
How to draw the potential energy diagram for this reaction ... 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
PDF Representing a Reaction with a Potential Energy Diagram Representing a Reaction with a Potential Energy Diagram (Student textbook page 371) 11. Complete the following potential energy diagram by adding the following labels: an appropriate label for the x-axis and y-axis, E a(fwd), E a(rev), ΔH r. a. Is the forward reaction endothermic or exothermic? b.
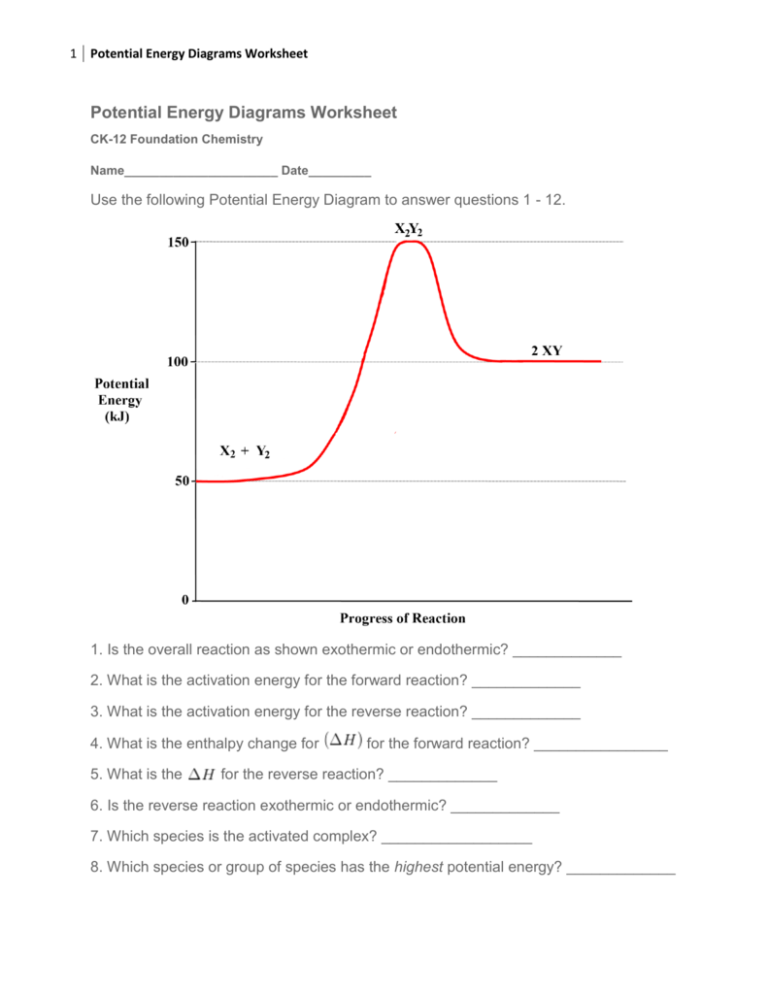
Welcome to CK-12 Foundation | CK-12 Foundation Exothermic and Endothermic Potential Energy Diagrams There are two types of potential energy diagrams. These two types center on the difference between the energies of the reactants and products. Consider the figure below. An endothermic reaction is shown on the left, and an exothermic reaction is shown on the right. ...
Endothermic Reactions: Definition, Example, Diagram and ... Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants.
potential_energy_diagrams_worksheet (1).docx - REACTION ... On the potential energy diagram indicate where the activated complex will be formed. Enthalpy Change As shown above, when reactions occur there will be a difference in potential energy between the reactants and products. This difference in potential energy is known as the enthalpy change and is given the symbol ΔH. The units for ΔH are kJ mol -1 .
Endothermic and Exothermic Reactions With Potential Energy ... This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams....
What are Endothermic Reactions? (with Examples & Video) The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant.






















0 Response to "40 potential energy diagram endothermic"
Post a Comment