39 draw the octahedral crystal field splitting diagram for each metal ion.
exercises - transition metals and coordination chemistry - chemistry the... Draw the crystal-field energy-level diagram for the d orbitals of an octahedral complex, and show the placement of the d electrons for each 2+ ion, assuming a strong-field complex. Diagram the splitting of the metal d orbitals that would result from such a crystal field. Assuming a strong field... PDF Outline of crystal field theory Crystal field splitting in octahedral coordination. Figure 2.5 Schematic molecular orbital energy level diagram for a transition metal coordination cluster, [ML6]. (a) Energy Electronic configurations and crystal field stabilization energies of the transition metal ions in high-spin and low-spin states in...
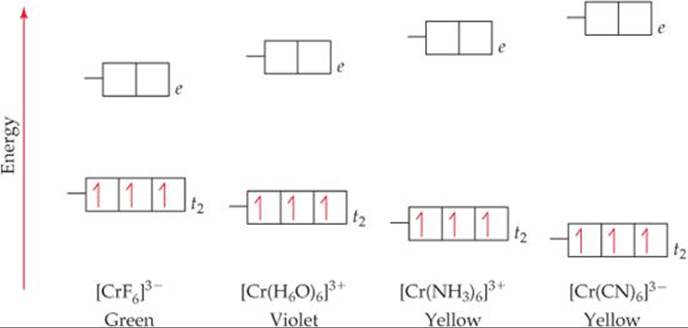
Transition Chemistry | studyslide.com Instead, we turn to crystal field theory, which highlights the effects on the d-orbital energies of the metal ion as a ligand approaches to form a coordinate For each of the two octahedral complex ions [Fe(H2O)6]2+ and [Fe(CN)6]4-, draw an orbital splitting diagram, predict the number of unpaired...

Draw the octahedral crystal field splitting diagram for each metal ion.
Crystal field theory. Tetrahedral and square planar geometries Trick for Crystal field theory (CFT) of Octahedral & Tetrahedral complexes | Coordination Compounds. Using the Spectrochemical Series to draw a metal complex ion's crystal field splitting. Crystal Field Theory (CFT). When applied to alkali metal ions containing a symmetric sphere of charge, calculations of bond energies are That is, the exact opposite of the situation we just dealt with for the octahedral crystal field. Note that a different CFT energy splitting diagram has to be applied for each stereochemistry. Draw the octahedral crystal field splitting diagram for each ... Answer to: Draw the octahedral crystal field splitting diagram for each metal ion. a. C r 3 + b. C u 2 + c. M n + (high- and low-spin) d. ...1 answer · Top answer: (a.) In Cr3+Cr3+, there are 3 electrons in the 3d orbital. The crystal field splitting diagram for Cr3+Cr3+ metal ion is, ...
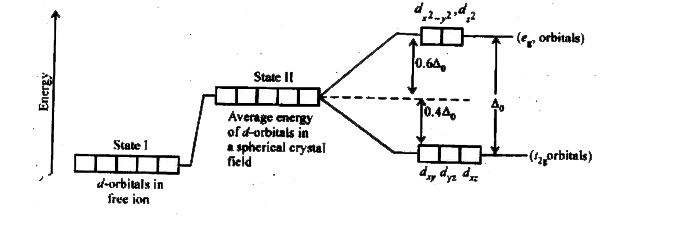
Draw the octahedral crystal field splitting diagram for each metal ion.. Orbital diagram for the metal orbitals in the octahedral Crystal Field Crystal Field Theory. Let us consider an octahedral arrangement of ligands around the central metal ion. For the octahedral field generated, the ligands are considered to be point charges sited on the cartesian axes, and the effect these point charges have on the valence orbitals of the metal ion is... Crystal Field Theory | Octahedral Crystal Fields Octahedral Crystal Fields. Each Mn2+ ion in manganese(II) oxide is surrounded by six O2- ions arranged toward the corners of an octahedron, as shown in the figure below. MnO is therefore a model for an octahedral complex in which a transition-metal ion is coordinated to six ligands. Draw figure to show splitting of d orbitals in an octahedral crystal field. Crystal field effects in octahedral coordination entities: (i) Let us assume that the six ligands are positioned symmetrically along the cartesian axes, with metal atom at the origin. As the ligands approach first there is an increase in energy of d-orbitals relative to that of the free ion just as would... Resolve a DOI Name Type or paste a DOI name into the text box. Click Go. Your browser will take you to a Web page (URL) associated with that DOI name. Send questions or comments to doi ...
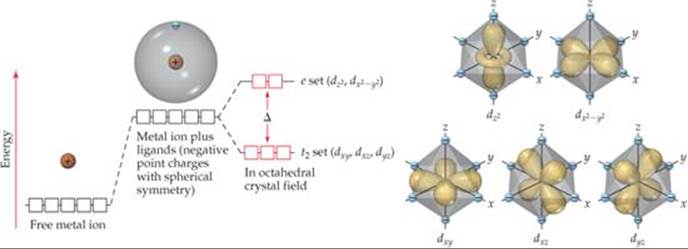
Draw the octahedral crystal field splitting diagram for each ... So the octahedron from crystal field splitting takes place like this two off. The orbital's going higher and adjusted, and in We call them Tito cheer pickles. And this as each your vittles. So for chromium three plus, we have for chromium. Spin crystal filled orbital spitting diagram for em in Tripolis looks like and then for if it topless we has we have ah ah Transition Metals and Coordination Compounds. Crystal Field Theory To understand how crystal field theory explains the electronic structures and colors of metal Placing a charge of −1 at each vertex of an octahedron causes the d orbitals to split into two groups with orbitals are placed in an octahedral crystal field. (Δo), where the subscript o stands for octahedral. Crystal Field Splitting - an overview | ScienceDirect Topics Crystal field d orbital splitting diagrams for common geometries. A splitting of magnitude Δ7 is produced and it depends on both the nature of the metal ion and of the ligand. In the case of the octahedral field each electron placed in one of the t2g orbitals is stabilised by a total of 2/5Δ, while... Draw The Octahedral Crystal Field Splitting Diagram For Each... How to draw the crystal field splitting diagram 2. Crystal field splitting energy 4. Cfse the stability that results from placing a transition metal ion in the crystal field generated by a set of Home question construct the octahedral crystal field splitting diagram for the metal in each species.
(PDF) Inorganic Chemistry 4th edition ... - Academia.edu Inorganic Chemistry 4th edition, Catherine Housecroft. 2012. Thang Pham Solved Draw the octahedral crystal field splitting diagram Chemistry questions and answers. Draw the octahedral crystal field splitting diagram for each metal ion. Cr3+ Cu2+ Mn3+ (high-spin) Mn3+ (low-spin) Fe2+ (low-spin). Crystal field theory - Wikipedia In a tetrahedral crystal field splitting, the d-orbitals again split into two groups, with an A compound that has unpaired electrons in its splitting diagram will be paramagnetic and will be attracted by Octahedral crystal field stabilization energy. Degenerate atomic d-orbitals of a free metal ion (left)... Bonding in Coordination Compounds: Crystal Field Theory Octahedral CFT splitting: Electron diagram for octahedral d shell splitting. Crystal field stabilization is applicable to the transition-metal complexes of Therefore, the crystal field splitting diagram for square planar geometry can be derived from the octahedral diagram. The removal of the two ligands...
1s2p RIXS Calculations for 3d Transition Metal Ions in Octahedral... We discuss the crystal field effects and the selection rules with respect to the 1s2p RIXS pre-edge and compare their final state energies with the total angular momentum IRREP as the ground state for the example 3d8 in an octahedral crystal field. To summarise all this information in a condensed form...
Lecture 9 - Crystal field theory for octahedral, tetrahedral and square... If the metal ion has electrons in its d-orbitals, it can donate them to the phosphine ligand through The orbital splitting diagram for square planar coordination can thus be derived from the octahedral 13 The crystal field stabilization energy for a diamagnetic square planar d 8 metal complex is readily...
Answered: 12. Draw the octahedral crystal field… | bartleby Q: Complete and balance each of the given nuclear equations by supplying the missing particle. Q: What are the ion concentrations for Ca2+ and NO3- in 0.35M Ca(NO3)2? A: We are having the Concentration of the Ca(NO3)2 as 0.35 M We have to calculate the concentration of...
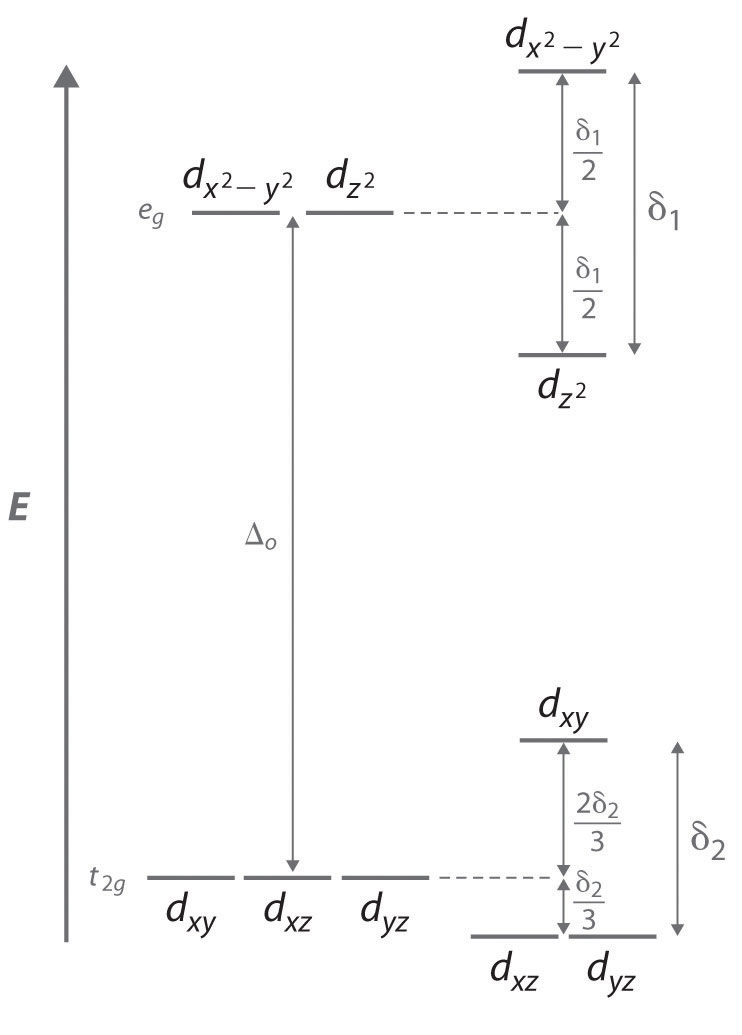
How do p-orbitals and d-orbitals split in an octahedral crystal field? Crystal field splitting for octahedral complex is: So, because of above reason in dx2-y2 and dz2 there is repulsion between the orbitals and ligands and it goes higher in You can calculate crystal field splitting energy by using above diagram for various complexes with six number of ligands present.
(PDF) Crystal Field Splitting in an Octahedral Field - Academia.edu Crystal Field stabilization Energy Factors Affecting the Magnitude of Δ Factors Affecting the Magnitude of Δ Factors Affecting the Magnitude of Δ Geometry of the metal coordination unit affects ∆ greatly. Tetrahedral complexes ML4 have smaller ∆ than octahedral ones ML6: ∆= 10,200 cm-1 for [CoII...
42. Draw the octahedral crystal field splitting diagram for each... 42. Draw the octahedral crystal field splitting diagram for each metal ion. a. Cr3+ b. Cu2+ c. Mn+ (high- and low-spin) d. Fe2+ (low-spin).
Construction of Pyrazine-Appended 1D and 3D Cobalt(II ... 10-02-2022 · The field-dependent magnetization studies, viz., zero-field cooling (ZFC) and field cooling (FC), were performed between temperatures 60 and 310 K under an applied magnetic field of 100 G. The variable-temperature magnetic susceptibility data for only 1 was obtained on a vibrating sample magnetometer (VSM) with the sensitivity of a SQUID (Superconducting …
Draw the octahedral crystal field splitting diagram ... - StudySoup Draw the octahedral crystal field splitting diagram for each metal ion. a. Cr3+ b. Cu2+ c. Mn3+ (high- and low-spin) d. Fe2+ (low-spin)1 answer · Top answer: SPRING 2016 CHEM 112 MONTANO Chapter 1 Matter Properties of matter are determined by the properties of the molecules and/or atoms it is composed ...
PDF Principles of Chemical Science, Solutions for Lecture 28: Transition... 1. For each of the following ions, (i) draw an crystal field splitting diagrams to show orbital occupancies in both weak and strong octahedral fields Briefly explain your answer. (d) Estimate the octahedral crystal field splitting energy (Δo) in joules/mol if the wavelength most intensely...
Construct the octahedral crystal-field splitting diagram for the... The diagram shows a scale drawing of a lacrosse field. Based on crystal field theory, which of the following metal ions will not be colored when placed in an octahedral crystal field? A d1 octahedral complex is found to absorb visible light, with the absorption maximum occcurring at 523...
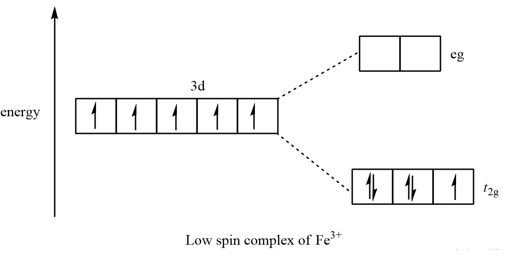
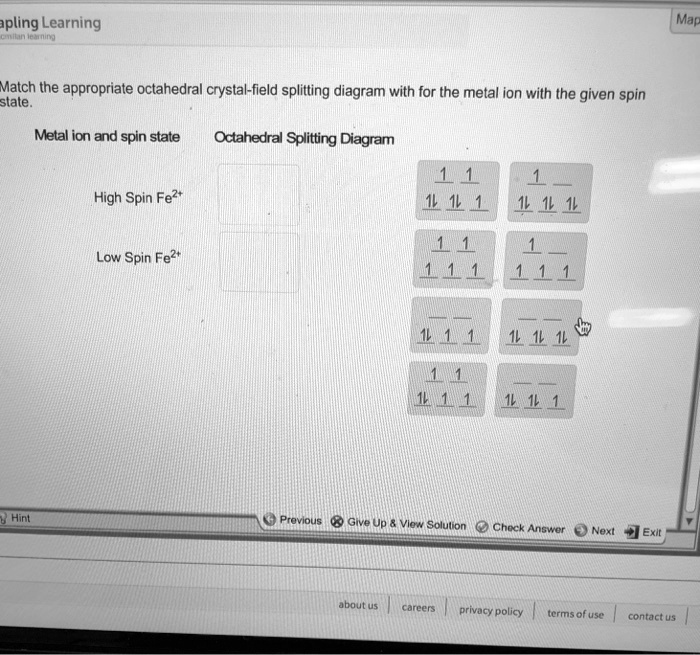
4.3: High Spin and Low Spin Complexes - Chemistry LibreTexts 28-01-2022 · Crystal Field Splitting Electron Count. In order to make a crystal field diagram of a particular coordination compound, one must consider the number of electrons. This can be done simply by recognizing the ground state configuration of the electron and then adjusting the number of electrons with respect to the charge of the metal.
PDF PowerPoint Presentation | octahedral crystal field The octahedral crystal field. ╪ Consider a first row metal cation surrounded by six ligands placed ╪ Each ligand is treated as a negative point charge and there is an electrostatic attraction between the 63. Approximate partial MO diagrams for metal-ligand p-bonding in an octahedral complex with...
Crystal Field Theory The relationship between colors and complex... 2 Crystal Field Model A purely ionic model for transition metal complexes. Ligands are considered as point charge. Predicts the pattern of splitting of The electrons of the central metal ion and those of the ligands have repulsive effect. This causes the splitting of the degenerate d orbitals into two...
Draw figure to show the splitting of d orbitals in an octahedral crystal... > The crystal field splitting for Cr3+ ion in octahedral field changes for following ligands in decreasing order as > Draw energy level diagrams and indicate the occupancy of the orbitals in the following complexes : (a) d6 octahedral, low-spin (b) d9 octahedral with tetragonal elongation (c) d8...
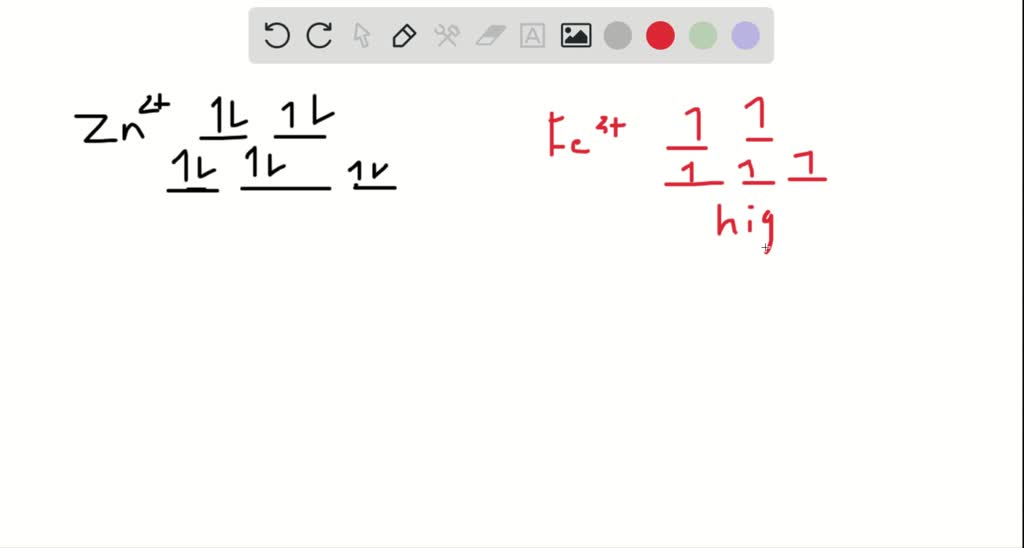
Draw the octahedral crystal field splitting diagram... | Course Hero 19. Draw the octahedral crystal field splitting diagram for the following metal ions:(a) Zn2+(b) Fe3+(high and low spin).
Introduction to Inorganic Chemistry/Coordination Chemistry and... Coordination compounds (or complexes) are molecules and extended solids that contain bonds between a transition metal ion and one or more ligands. In forming these coordinate covalent bonds, the metal ions act as Lewis acids and the ligands act as Lewis bases.
CHEM2P32 Lecture 11. Square and Tetrahedral Complexes The orbital splitting diagram for square planar coordination can thus be derived from the octahedral diagram. As ligands move away along the z-axis, d-orbitals with a 2. Crystal Field Stabilization Energy in Square Planar Complexes. Square planar coordination is rare except for d8 metal ions.
Crystal Field Theory - Chemistry LibreTexts In Crystal Field Theory, it is assumed that the ions are simple point charges (a simplification). When applied to alkali metal ions containing a symmetric sphere of charge, calculations of bond energies are generally quite successful. The approach taken uses classical potential energy equations that take...
Draw the octahedral crystal field splitting diagram for each ... Answer to: Draw the octahedral crystal field splitting diagram for each metal ion. a. C r 3 + b. C u 2 + c. M n + (high- and low-spin) d. ...1 answer · Top answer: (a.) In Cr3+Cr3+, there are 3 electrons in the 3d orbital. The crystal field splitting diagram for Cr3+Cr3+ metal ion is, ...
Crystal Field Theory (CFT). When applied to alkali metal ions containing a symmetric sphere of charge, calculations of bond energies are That is, the exact opposite of the situation we just dealt with for the octahedral crystal field. Note that a different CFT energy splitting diagram has to be applied for each stereochemistry.
Crystal field theory. Tetrahedral and square planar geometries Trick for Crystal field theory (CFT) of Octahedral & Tetrahedral complexes | Coordination Compounds. Using the Spectrochemical Series to draw a metal complex ion's crystal field splitting.
























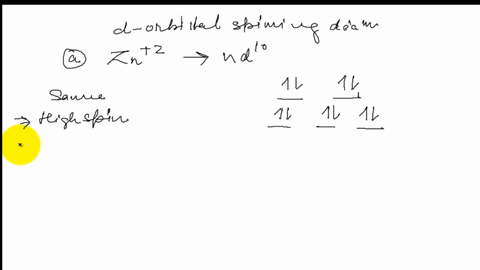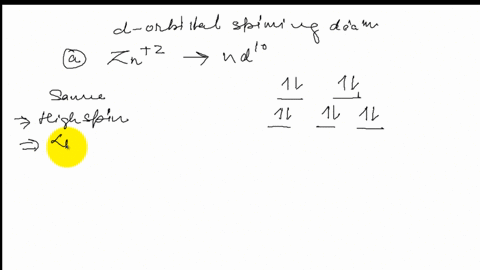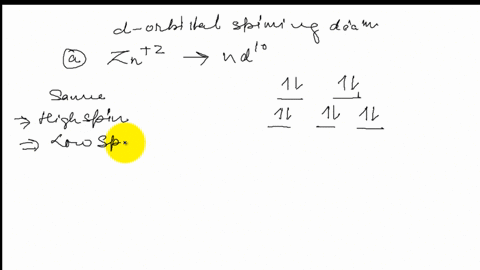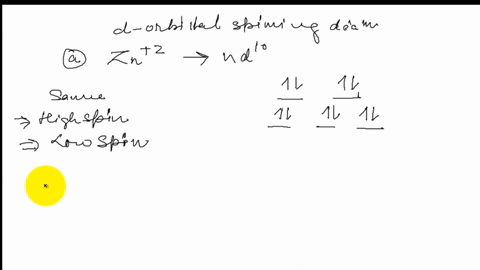
0 Response to "39 draw the octahedral crystal field splitting diagram for each metal ion."
Post a Comment