36 lewis diagram for o2
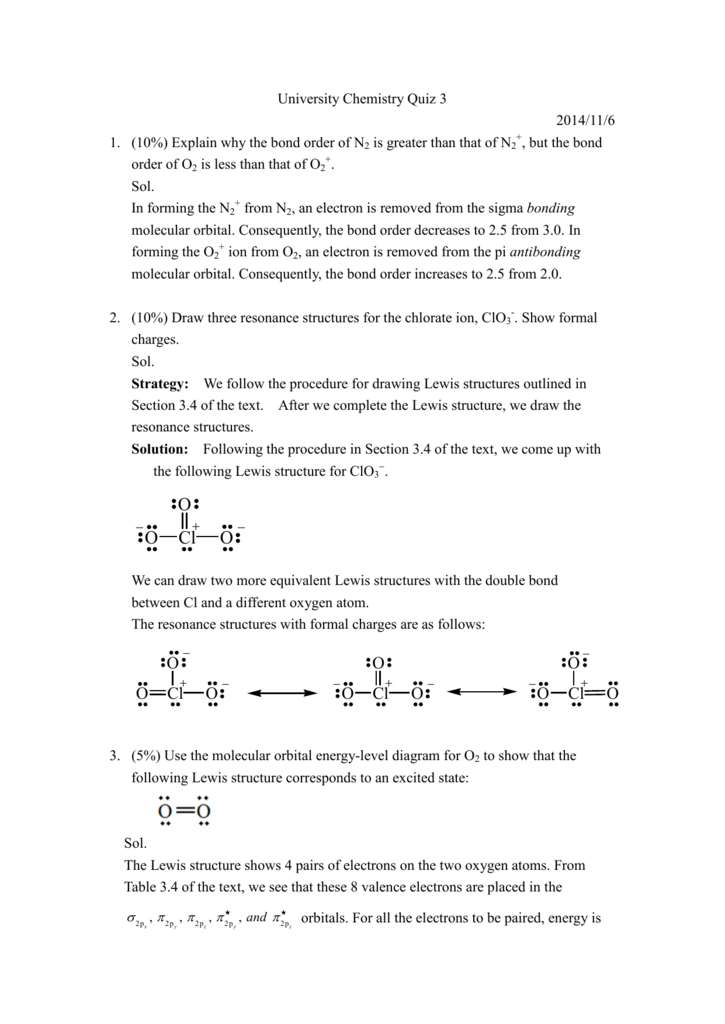
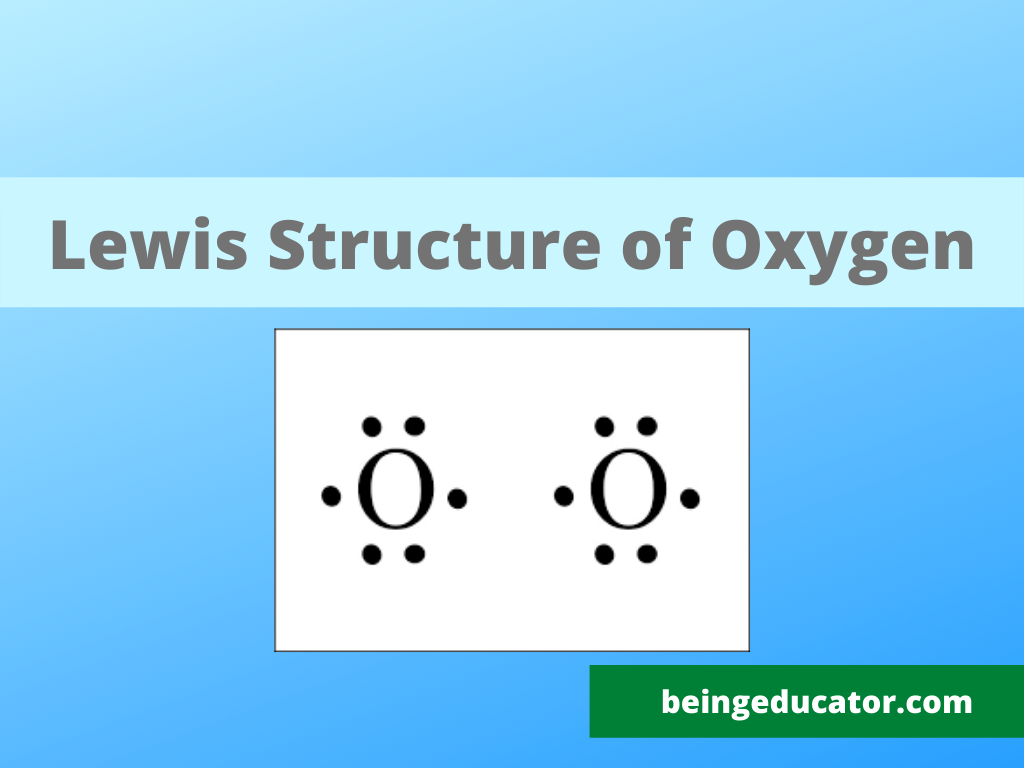
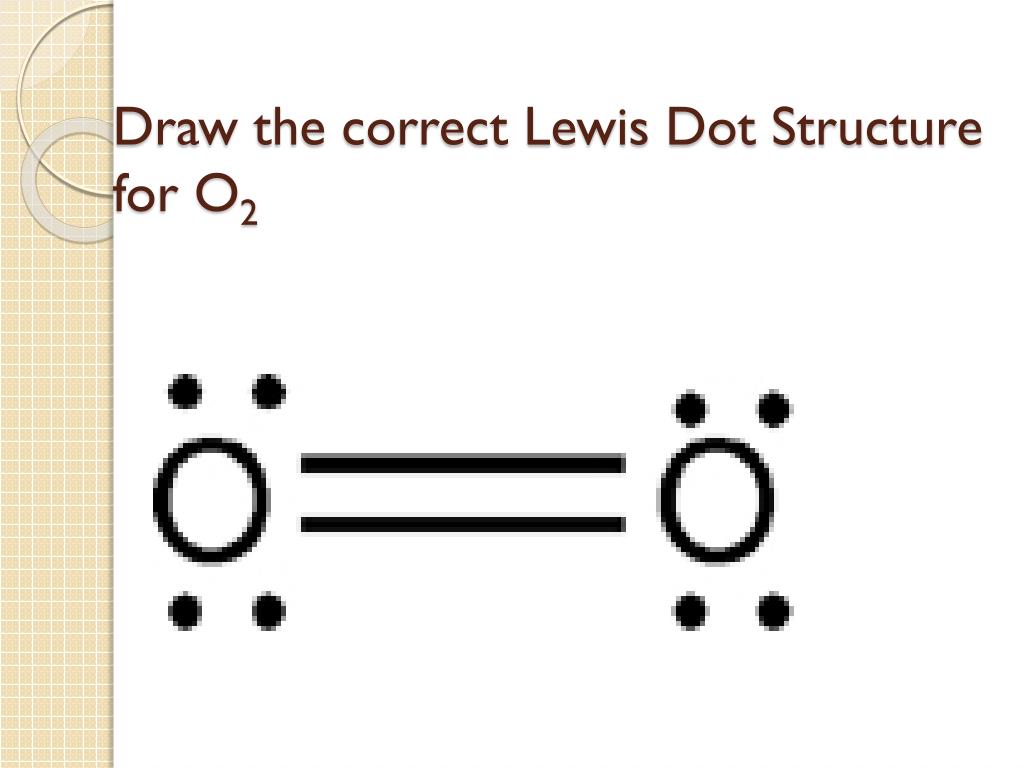
The Lewis diagram for N 2 is as follows: The total number of electrons is 4 x 2 (1) + 6 = 12 electrons. In CH 2 O, the central atom is surrounded by two different types of atoms. The Lewis diagram that fills each atom's valence electron shell is as follows: Exercise 4.3. 1. Draw the Lewis diagram for each molecule. O2 Lewis Structure. O2 Lewis structure, oxygen is the diatomic molecule and hence two atoms of the elements combine together to form dioxygen. The oxygen has six electrons in its valance shell. They try to gain or share two electrons to complete their octet and to get stability. By using the formula of Q, we can calculate the total electrons
Lewis Dot Diagram For Oxygen. Oxygen has a special rule when doubling. Lewis dot diagrams displaying higher than optimal formal charges represent higher energy states of the species. Lewis dot structures for the first few non-‐metals. The lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared ...
Lewis diagram for o2
The Lewis structure gives oxygen an octet and each hydrogen 2 electrons. Example \(\PageIndex{2}\) Write the Lewis structure for the \(CH_2O\) molecule. Solution. Steps for Writing Lewis Structures. Example \(\PageIndex{2}\) 1. Determine the total number of valence electrons in the molecule or ion. 5.3: Lewis Diagrams. Lewis used simple diagrams (now called Lewis diagrams) to keep track of how many electrons were present in the outermost, or valence, shell of a given atom. The kernel of the atom, i.e., the nucleus together with the inner electrons, is represented by the chemical symbol, and only the valence electrons are drawn as dots ... A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
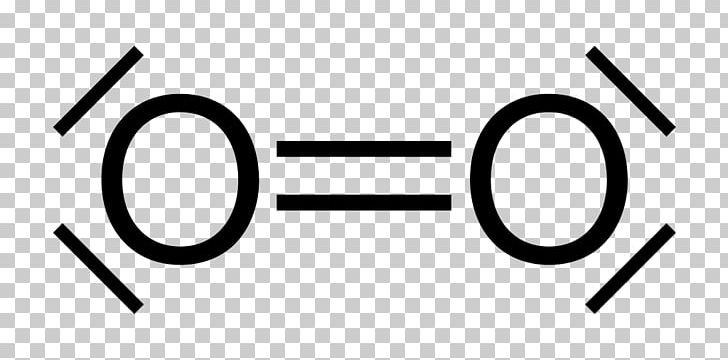
Lewis diagram for o2. Oxygen Lewis Dot Structure. Oxygen Lewis Structure. Lewis Structure O2 * Please keep in mind that all text is machine-generated, we do not bear any responsibility, and you should always get advice from professionals before taking any actions. Sources What is the Lewis structure for Oxygen (O2) A. A. B. B. C. C. D. D. 3. What is the Lewis formula for ammonia (NH 3)? A. A. B. ... electron dot structures, Lewis electron dot structures, Lewis dot structures, Lewis dot diagrams, or Lewis dot formulas, it wouldn't matter because the use is exact. They are diagrams... Questions: 10 | Attempts: 436 ... Oxygen (O2) is a commonly tested Lewis structure due to it's importance on Earth. It also is a good example of a molecule with a double bond. There are 12 ...26 Oct 2016 · Uploaded by Wayne Breslyn To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s22s22p6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level.
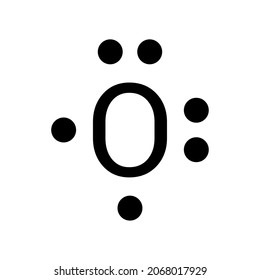
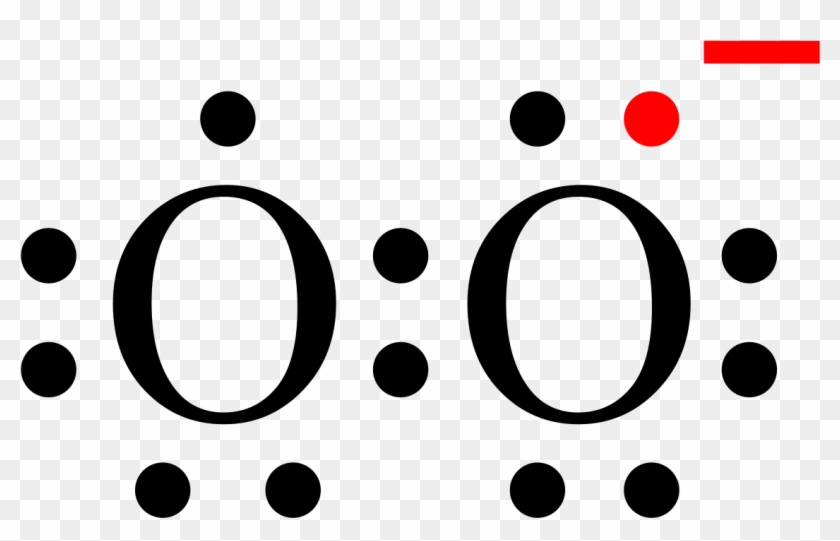
15 Ch4O Lewis Structure. Remember that hydrogen atoms always go on the outside of a lewis structure and that they only need two valence electrons for a full outer shell. The xe atom is attached to all four oxygen atoms. Worksheet 1 - solutions A - CHEM 2400 Video 1-2… 13+ H2 Lewis Structure. You have a total of 8 valence electrons available to fill the octets of oxygen and hydrogen. All valence electrons of the atoms in lewis structures must be shown. H2S Lewis Structure - How to Draw the Dot Structure for … from i.ytimg.com. Most lewis structures you encounter will be covalent bonds. XeO4 Lewis Structure, Geometry, Hybridization, and Polarity. XeO4 or Xenon Tetraoxide is a chemical compound made up of Xenon and Oxygen. It is prepared by treatment of barium perxenate with anhydrous sulphuric acid. It has a molar mass of 195.29 g/mol. It is exceptional for being a stable compound of a noble gas comprising of Xenon in its ... The Lewis structure helps with understanding how electrons are distributed within a compound along with its molecular geometry. Besides this, the lewis structure helps with determining the hybridization of the molecule. Lewis structure of O2. The Lewis diagram of O2 shows two oxygen atoms having twelve dots, of valence electrons.
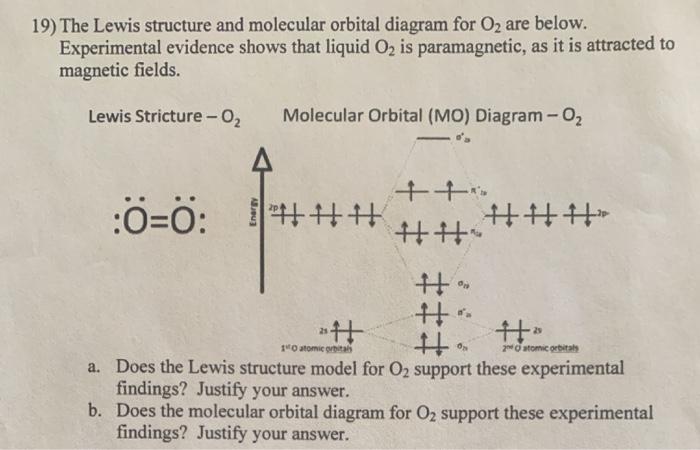
The Lewis structure of the triatomic H2O molecule shows two single sigma bonds between the oxygen atom and the hydrogen atoms. Moreover, these bonds leave two lone pairs of electrons on the oxygen atom that mainly contributes to the tetrahedral bent geometrical structure of the H2O molecule. Hello, So I am currently reading up about bonding and anti-bonding orbitals in Molecular Orbital Theory and am very confused as to how they relate to the actual bonds. I see you can use the Bond Order Formula to determine HOW MANY bonds the compound will have, but I don't see how the different orbitals actually relate to the bonds a compound makes, like what orbitals are doing what and which bond is formed by which orbital or whatever. For example, I am looking at a MO diagram of O2 and I get ... Lewis Structure of Carbon Dioxide ( C O 2). In ( C O 2), oxygen belongs to group 16 of the Periodic Table, and carbon belongs to group 14 of the Periodic Table. Hence, oxygen has 6 valence electrons, and carbon has 4 valence electrons. Step 1- In ( C O 2) we, have C = 4 × 1 = 4 valence electrons. The CO 2 Lewis structure has two double bonds going from carbon to the oxygen atoms. According to the octet rule, each oxygen atom needs to bond twice and the carbon atom needs to bond four times. What is the best Lewis structure for CO2?, Lewis structure for carbon dioxide Skeletal structure.
Moreover, these eight electrons are drawn only around the prize of the atom in the Lewis structure. The oxygen has actually a dearth of two valence electrons. Whereas, the 2 hydrogen atoms have actually a dearth of two valence electrons in total. The Lewis framework of H2O is attracted in such a manner that the deficiency of each atom is fulfilled.
Let us follow some steps to draw the Lewis structure of chlorine dioxide: Step 1: Find the total valence electrons in one molecule of chlorine dioxide. It is 20 as chlorine has 7 valence electrons and oxygen has 6 valence electrons. There are two oxygen molecules in chlorine dioxide so the total is 19.
SeO2 Lewis Structure, Geometry, Hybridization, and Polarity. The chemical formula of selenium dioxide is SeO2. It is a unidimensional polymer chain having alternating selenium and oxygen atoms. This chemical compound is of great importance because of its corrosive nature for metals only when in contact with water.
The Lewis structure gives oxygen an octet and each hydrogen 2 electrons. Example \(\PageIndex{2}\) Write the Lewis structure for the \(CH_2O\) molecule. Solution. Steps for Writing Lewis Structures. Example \(\PageIndex{2}\) 1. Determine the total number of valence electrons in the molecule or ion.
Each oxygen in the SiO2 lewis dot structure contains two lone pairs of electrons and 2 bonding pairs of electrons whereas silicon has zero lone pairs and 4 bonding pairs of electrons same as the CO2 lewis diagram.
Based on the formal charges of the chlorine and oxygen atoms, Lewis structure for can be drawn as shown below. The most preferred Lewis structure for is as follows: Formal charges of chlorine, negatively charged, and double bonded oxygen are 0,-1, and 0 respectively. They can be indicated as shown below.
The Lewis dot diagram shows two oxygen atoms sharing two pairs of electrons (forming a double bond). Each of the atoms also has two lone pairs (electrons that ...4 answers · 19 votes: Oxygen atoms have 6 valence electrons. They need a stable octet but forming one bond wouldn't ...
14+ Lewis Dot Structure For O2. We use lewis dot structures based on valence electrons. The lewis dot structure for o2 or dioxygen is as follows: Lewis structure is basically a graphic representation of the electron distribution around an atom. You must draw the lewis dot structure first.
The O2 Lewis structure has a double bond between two oxygen atoms. According to the octet rule, oxygen atoms need to bond twice. The O2 molecule is diatomic ...
Lewis structure diagrams a part of prerequisite expectations, but I've never been taught how to do them. Anyways, why is it when we draw the Lewis Structure for O2 in the video, you move the electrons to share when there is already a lone pair created? And how can you leave the top of the atom vacant of electrons? Video: https://www.youtube.com/watch?v=pC_-KW6NcvA
Answer (1 of 3): https://learnwithdrscott.com/o2-lewis-structure/ The two letter O's in the O2 Lewis structure represent the nuclei (centers) of the oxygen atoms. The ...
H2O2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. H2O2 is a chemical compound with the IUPAC name Hydrogen Peroxide. It is the simplest peroxide compound, i.e., a molecule containing an Oxygen-Oxygen single bond. It is a pale blue liquid in its standard state and slowly reacts with sunlight and decomposes into water and oxygen.
The Lewis structure for oxygen O2 shows the bond between two oxygen atoms. Drawing the Lewis Structure for OF2. Lewis dot diagram can be drawn for element simple ions polyatomic ions ionic and molecular compounds. When drawing the structure you may replace the individual lines with two dots symbolizing the two electrons contained within the.
Answer (1 of 3): I modified the picture from this post: What's the MOT diagram of O2 +2 ion? and modified it to be O2 2+ (since sadly enough I am about as advanced with artistic programs on pc as a rock). How you basically do these questions is by first drawing the empty AO and MO, then counting ...
By analyzing the Lewis structure of SO2, we can see that the SO2 is asymmetrical because it contains a region with different sharing. The molecular geometry of SO2 has a bent shape which means the top has less electronegativity, and the bottom placed atoms of Oxygen have more of it. So, the conclusion is, SO2 is a Polar molecule.
With SO2 Lewis Structure, the central atom is the central sulfur atom because of the higher valence of the sulfur atom than the oxygen atom. The SO2 Lewis Structure provides the best explanation of how the sulfuric acid (1) transformed into such after dissecting the bonds of Sulfur and Oxygen. This can be a hazard to one's health, but this is ...
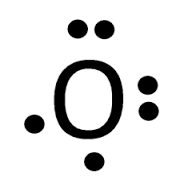
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
5.3: Lewis Diagrams. Lewis used simple diagrams (now called Lewis diagrams) to keep track of how many electrons were present in the outermost, or valence, shell of a given atom. The kernel of the atom, i.e., the nucleus together with the inner electrons, is represented by the chemical symbol, and only the valence electrons are drawn as dots ...
The Lewis structure gives oxygen an octet and each hydrogen 2 electrons. Example \(\PageIndex{2}\) Write the Lewis structure for the \(CH_2O\) molecule. Solution. Steps for Writing Lewis Structures. Example \(\PageIndex{2}\) 1. Determine the total number of valence electrons in the molecule or ion.


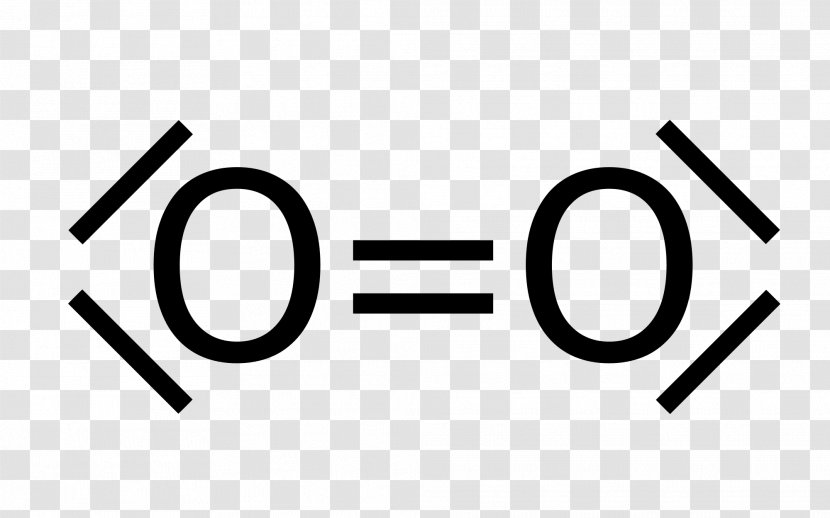







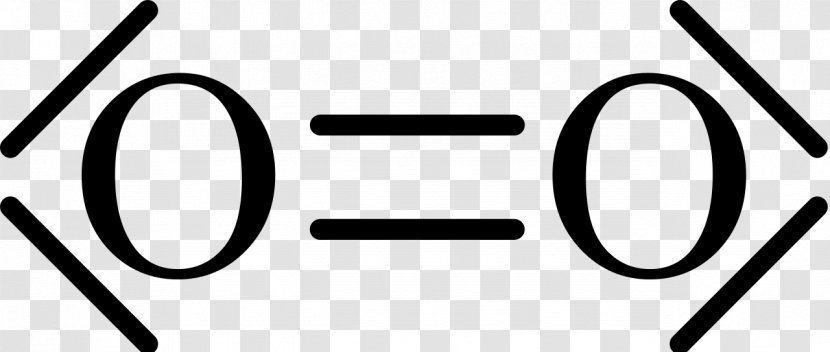










0 Response to "36 lewis diagram for o2"
Post a Comment