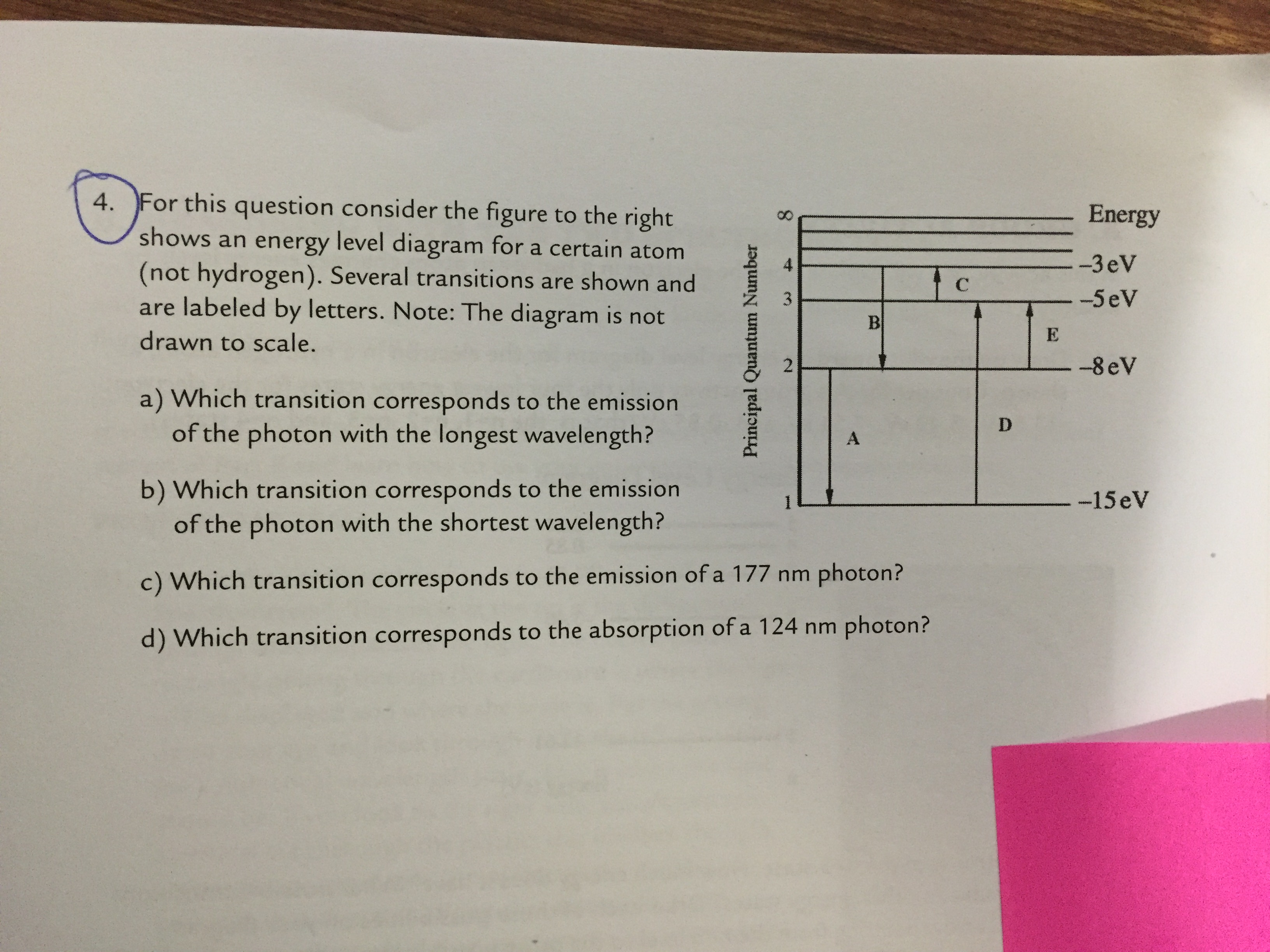
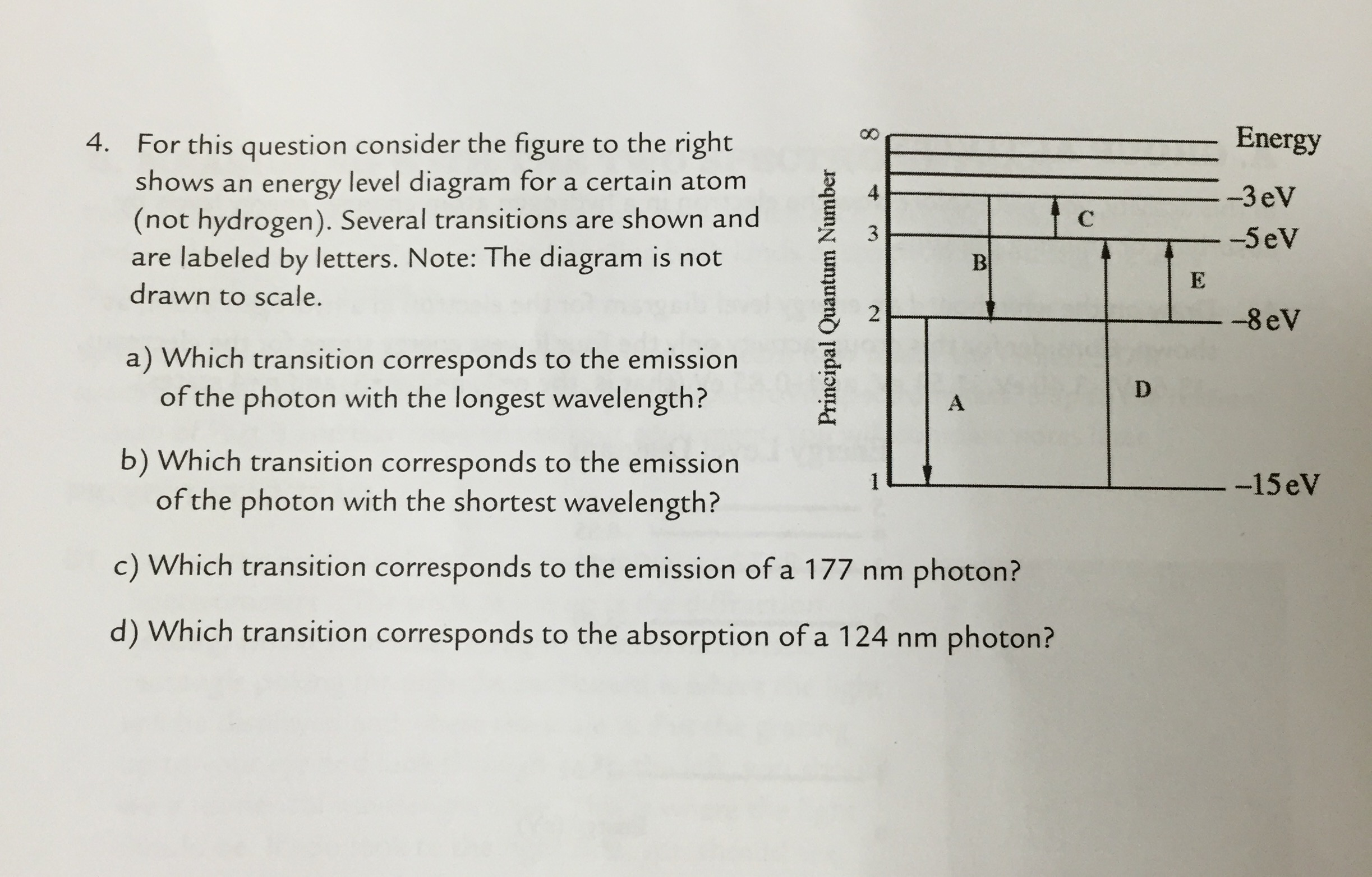
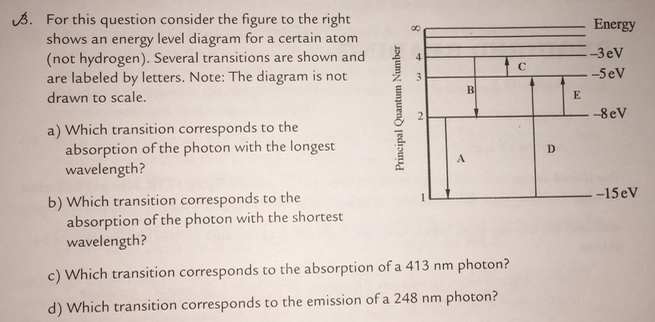
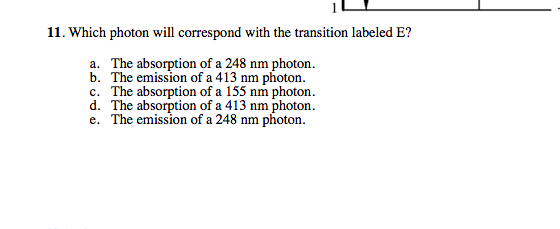
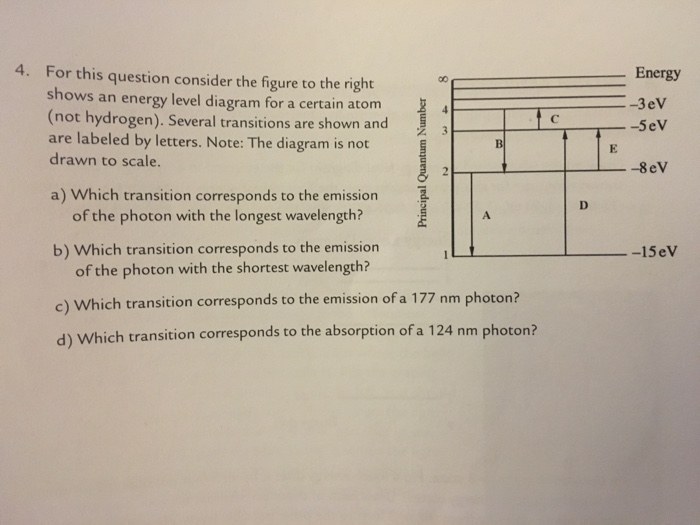
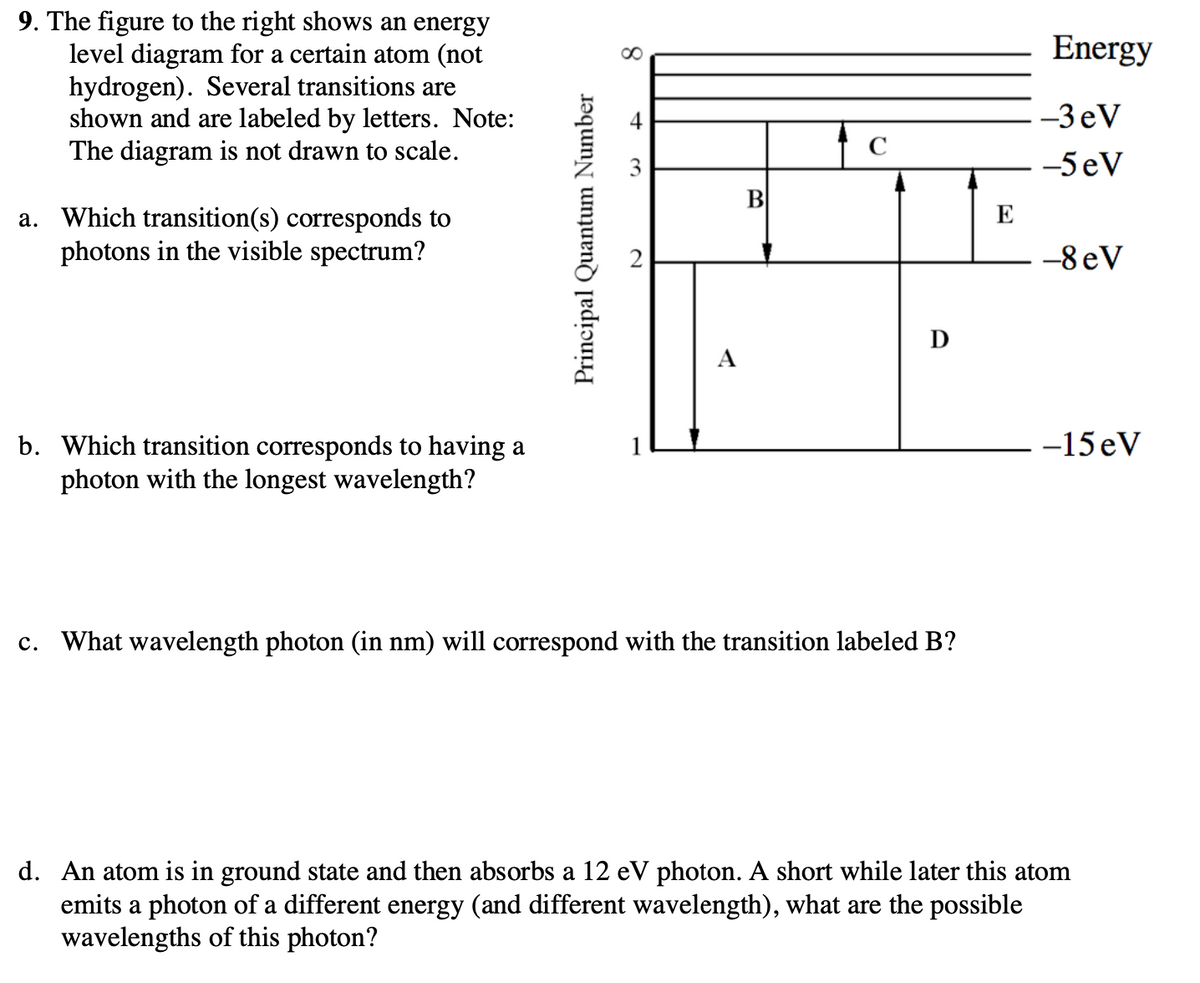
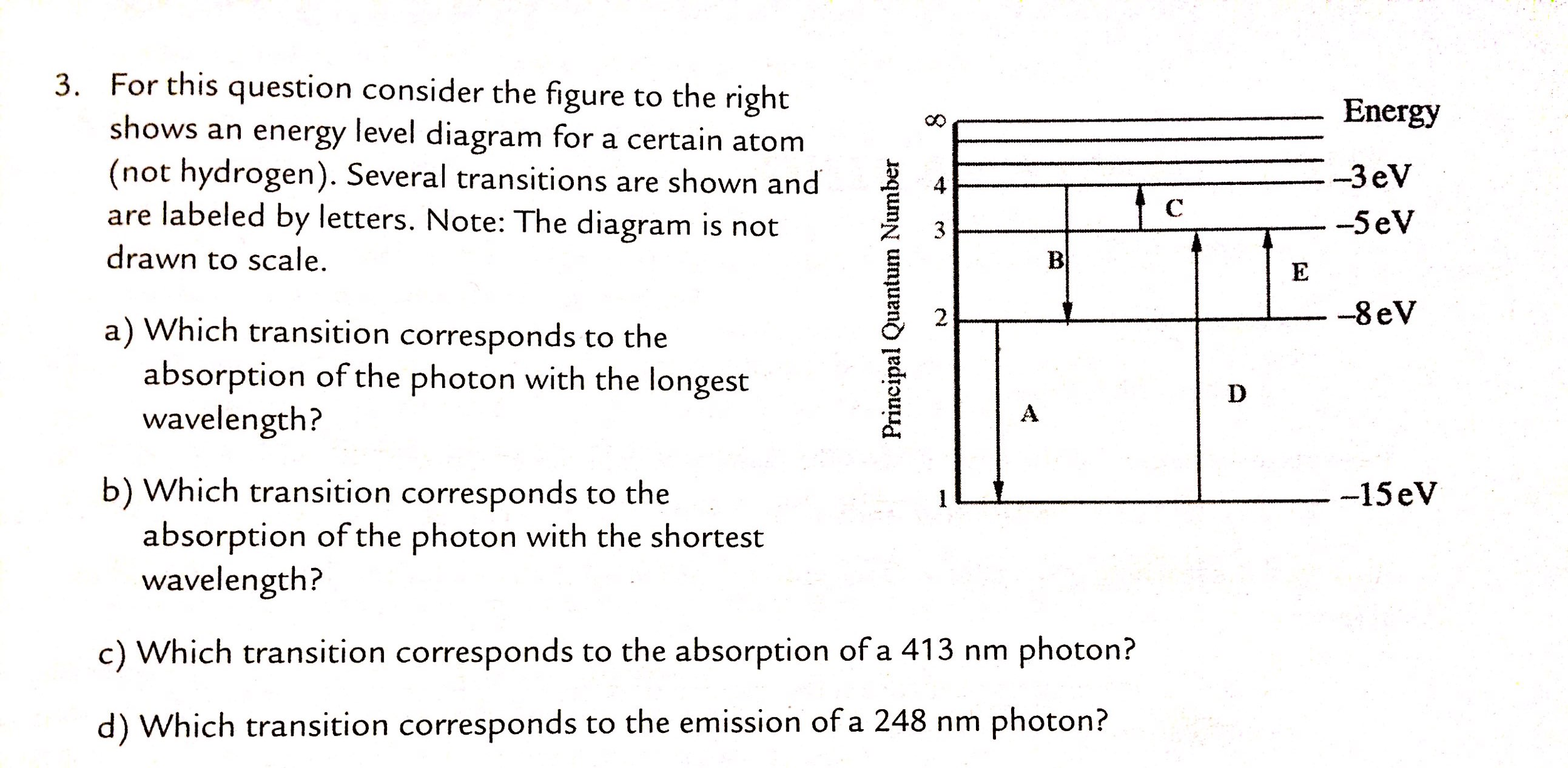
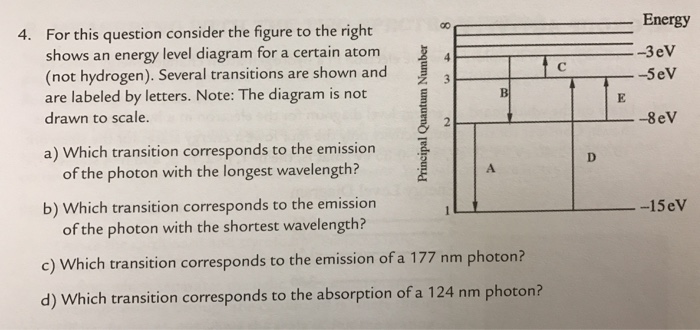
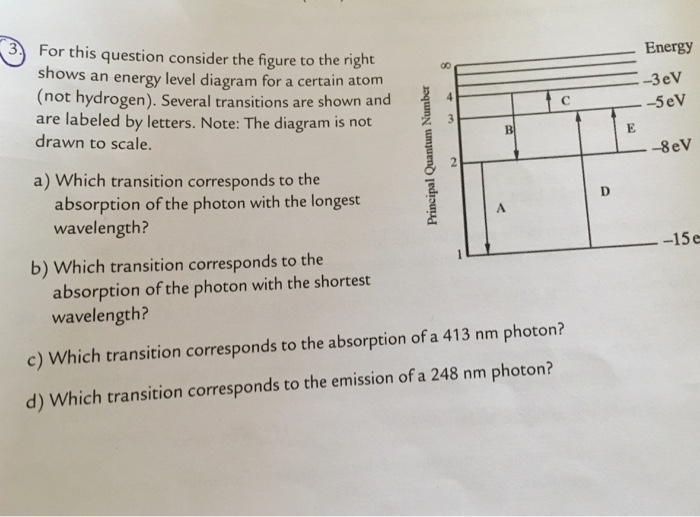
36 for this question consider the figure to the right shows an energy level diagram for a certain atom
We like representing these energy levels with an energy level diagram. The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Let's say our pretend atom has electron energy levels of zero eV, four eV, six eV, and seven eV.
Figure 4. This orbital energy-level diagram shows the sp hybridized orbitals on Be in the linear BeCl 2 molecule. Each of the two sp hybrid orbitals holds one electron and is thus half filled and available for bonding via overlap with a Cl 3p orbital. When atomic orbitals hybridize, the valence electrons occupy the newly created orbitals.
4.3.1 The Hydrogen atom In the previous chapter we studied stationary problems in which the system is best described as a (time-independent) wave, "scattering" and "tunneling" (that is, showing variation on its intensity) because of obstacles given by changes

For this question consider the figure to the right shows an energy level diagram for a certain atom
Sketch in Figure 3-6 both the total energy level of this car and ... atoms is to consider the potential energy created by the attraction, then analyze ... The diagram in Figure 3-8 shows potential and total energies for a car. Indicate on the graph the car's turning points.
Problem: For this question consider the Figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown ...1 answer · Top answer: The energy of a photon:E=hf=hcλEphoton = hc/λ = (6.62 × 10-34)(3.0 × 108)/(124 × 10-9) [readmore]Ephoton = 1.60 × 10-18 J(1eV/1.6 × 10-19J) = 10.0 ...
item select "Eigenvalue" Æ "Level Diagram". You should see an energy level diagram with two energy levels above and below a dotted line (similar to the one in Figure 5). The dotted line corresponds to α, the energy of the 2py atomic orbitals. The lower level is labeled with an energy of 1.00 and this corresponds to α + 1.00β.
For this question consider the figure to the right shows an energy level diagram for a certain atom.
The atom is in the ground state when it absorbs a photon with frequency 218 x 1015 hz. For this question consider the figure to the right shows an energy level diagram for a certain atom not hydrogen. Each orbit has its specific energy level which is expressed as a negative value.
Transcribed image text: Energy For this question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen).
For this question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are ...
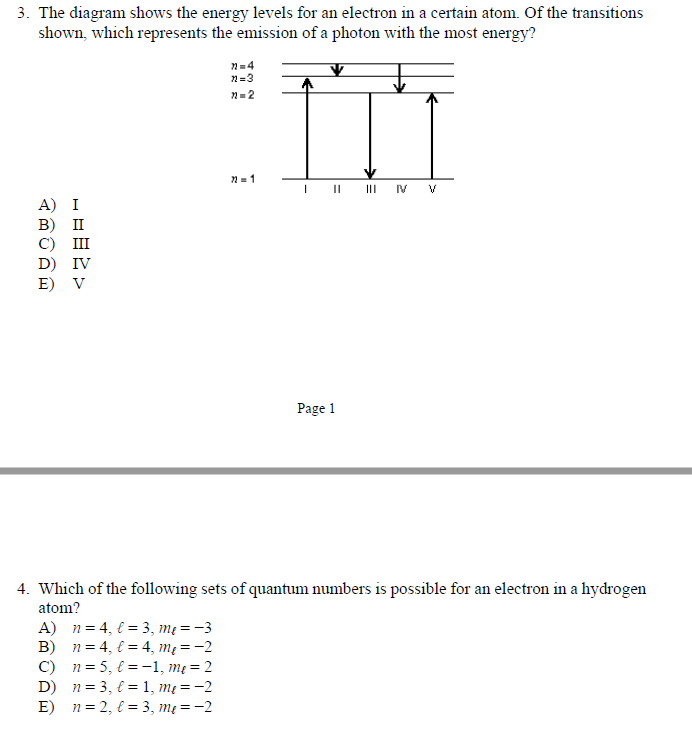
The figure shows an energy level diagram for the hydrogen atom. Several transitions are shown and are labeled by letters.Note: The diagram is not drawn to scale. Which transition corresponds to the absorption of the photon with the longest wavelength?
For this question consider the Figure to the right shows an energy level diagram for a certain atom
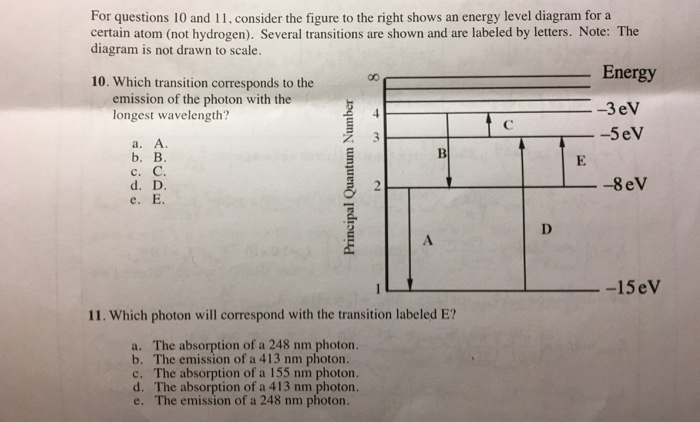
Figure 2 Refer to Figure 2 when answering questions #10 and #11. 10. In the two-level atom shown in Figure 2, which electron transition is associated with the emission of light? ANSWER. The transition labeled "b". 11. If the "c" transition marked in the three-level atom is associated with the absorption or
Circle the a propnate word to complete each statement in Questions 14—17. 14. Electrons and proton—epel) each other. to the nucleus gets ( tronger 15. As an electron gets closer to the nucleus weaker). 16. For an electron to move from an energy level close to the nucleus to an energy level far from the nucleus it would need teose) energy. 17.
Solution for Calculate the Energy! Student Worksheet Neils Bohr numbered the energy levels (n) of hydrogen, with level 1 (n=1) being the ground state, level 2 being the first excited state, and so on.Remember that there is a maximum energy that each electron can have and still be part of its atom. Beyond that energy, the electron is no longer bound to the nucleus of the atom and it is ...
Figure 2 shows the oscillation of the damped system after the wheel goes over a bump in the road. Figure 2 (i) Describe the energy changes that occur during the time covered by Figure 2, making reference to the types of energy involved at P, Q and R.
For this question consider the figure to the right that shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and labeled by letters. Note the diagram is not drawn to scale. (A) Which transition corresponds to the emission of the photon with the longest wavelength?
For certain atom,there are energy levels A,B,C corresponding to energy values E A < E B < E C .Choose the correct option if λ 1 , λ 2 , λ 3 are the wavelengths of radiations corresponding to the transition from C to B,B to A and C to A respectively.
Question: For This question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown ...
For this question consider the Figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Note: The diagram is not drawn to scale. Which transition corresponds to the emission of the photon with the longest wavelength? Which transition corresponds to the […]
(Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer.) 1._____contains all the electrons with similar energy. 2._____ is the region around the nucleus where electrons of a certain energy are most likely to be found.
Get the detailed answer: For this question consider the figure to the right shows an energy level diagram fora certain atom(not hydrogen). Several transiti
For this question consider the figure to the right that shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and labeled by letters. Note the diagram is not drawn to scale. (A) Which transition corresponds to the emission of the photon with the longest wavelength?
In the energy level diagram shown in the figure below the electron is excited to the e2 energy level. One star is red while the other star is blue. For this question consider the figure to the right shows an energy level diagram for a certain atom not hydrogen. You look on the periodic table and find that oxygen is atomic number 8.
For this question consider the figure to the right shows an energy level diagram for a certain atom not hydrogen. Macro exam 111213 the figure in the window on the right shows average annual growth rates in real gdp per hour worked in the united states. Ionization Energy Wikipedia One night you look up in the sky and notice that two of the brightest visible stars have distinctly different ...
What is energy level diagram? In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. The closest shell to the nucleus is called the "K shell" followed by the "L shell" then the "M shell" and so on away from the nucleus.
How many p electrons are there in an atom of rubidium? (a) 12 (b) 18 (c) 24 (d) 9 (e) 6 20. A neutral atom of an element has 2 electrons in the first energy level, 8 in the second energy level and 8 in the third energy level. This information does not necessarily tell us: (a) the atomic number of the element.
Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states.
Physics questions and answers. For this question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Which transition corresponds to the emission of the photon with the longest wavelength?
Pls help! For this question consider the Figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Note: The diagram is not drawn to scale. Which transition...
For this question consider the figure to the right shows an energy level diagram. Aa bb cc dd. D calculate the wavelengths for these photons. B sketch an energy level diagram to show the energy levels for n 2 through n 7 for hydrogen. The first electron goes into the 1s orbital filling the lowest energy level first and the second one spin pairs with the first one. More on pe diagrams. The ...
the fluorine atoms, (iii) from the carbon atom to a point mid-way between the hydrogen atoms, or (iv) From the carbon atom toward a hydrogen atom? [Sections 9.2 and 9.3] 9.5 The following plot shows the potential energy of two Cl atoms as a function of the distance between them. (a) To what does an energy of zero correspond in this diagram?
For this question consider the figure to the right shows an energy level diagram for a certain atom not hydrogen. Macro exam 111213 the figure to the right illustrates the relationship between weak and strong rule of law countries and economic growth. Supply curve upper s 1 represents the private cost of production and.
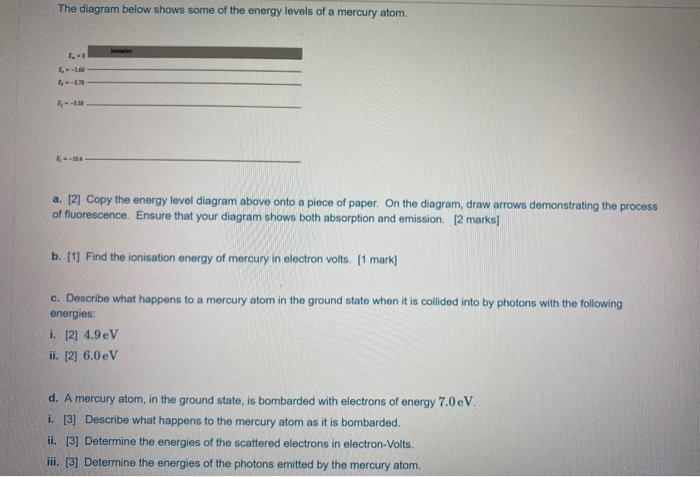
The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV. When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon.
For this question consider the figure to the right shows an energy level diagram for a certain atom. A which transition corresponds to the absorption of the photon with the longest wavelength. One night you look up in the sky and notice that two of the brightest visible stars have distinctly different colors. The diagram on the right is called an energy level diagram.
For this question consider the figure to the right shows an energy level diagram for a certain atom. A particular stars spectrum shows the same hydrogen line at a wavelength of 4860 nm. One night you look up in the sky and notice that two of the brightest visible stars have distinctly different colors. Each orbit has its specific energy level which is expressed as a negative value.
The diagram shows Niels Bohr's model of an atom. What happens when the electron moves from the first energy level to the second energy level? Energy is absorbed, and an emission line is produced. Energy is released, and an emission line is produced.[wrong] Energy is absorbed by the atom. Energy is lost from the atom.
Students will first look at a diagram and animation to understand the basic pattern of the arrangement of electrons on energy levels around an atom. Students will be given cards with information about the electrons and energy levels for each of the first 20 atoms. ... Tell students that this energy level model represents an atom. The nucleus is ...
Question: For this question consider the figure to the right shows an energy level diagram for a certain atom not hydrogen). Several transitions are shown ...
The excited state electron configuration of an atom indicates the promotion of a valence electron to a higher energy state. An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. Example The ground state electron configuration of sodium is "1s"^2"2s"^2"2p"^6"3s"^1. In its excited state, the valence electron in the "3s ...
Problem: For this question consider the figure that shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Note: The diagram is not drawn to scale. Which transition corresponds to the emission of the photon with the longest wavelength?
Question: For this question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Note: The diagram is not drawn to scale. Which transition corresponds to the emission of the photon with the longest wavelength?
Transcribed image text: For this question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen).
The figure shows the energy levels of an atom. The first four energy levels are shown here. The first energy level is also called level 'K'. The second level is called level L, third energy level as M, and so on. The electrons from energy level K contains the least energy whereas the levels that are far from the nucleus contains more energy
For this question consider the figure to the right shows an energy level diagram. Figure shows the energy level diagram for the reaction between methane and oxygen. If two electrons end up in the same orbital one arrow faces up and the other faces down. Based on figure the following information ...
For this question consider the figure to the right shows an energy level diagram. Several transitions are shown and are labeled by letters. Macro exam 111213 the figure in the window on the right shows average annual growth rates in real gdp per hour worked in the united states. This number means that oxygen has 8 protons in its nucleus and 8 electrons. You look on the periodic table and find ...
The equation also shows us that as the electron's energy increases (as n increases), the electron is found at greater distances from the nucleus. This is implied by the inverse dependence on r in the Coulomb potential, since, as the electron moves away from the nucleus, the electrostatic attraction between it and the nucleus decreases, and it is held less tightly in the atom.
In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. According to Bohr's theory, electrons of an atom revolve around the nucleus on certain orbits, or electron shells. Each orbit has its specific energy level, which is expressed as a negative value. This is because the electrons on the orbit are "captured ...
Physics. Physics questions and answers. For this question consider the Figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Note: The diagram is not drawn to scale. Which transition corresponds to the ...
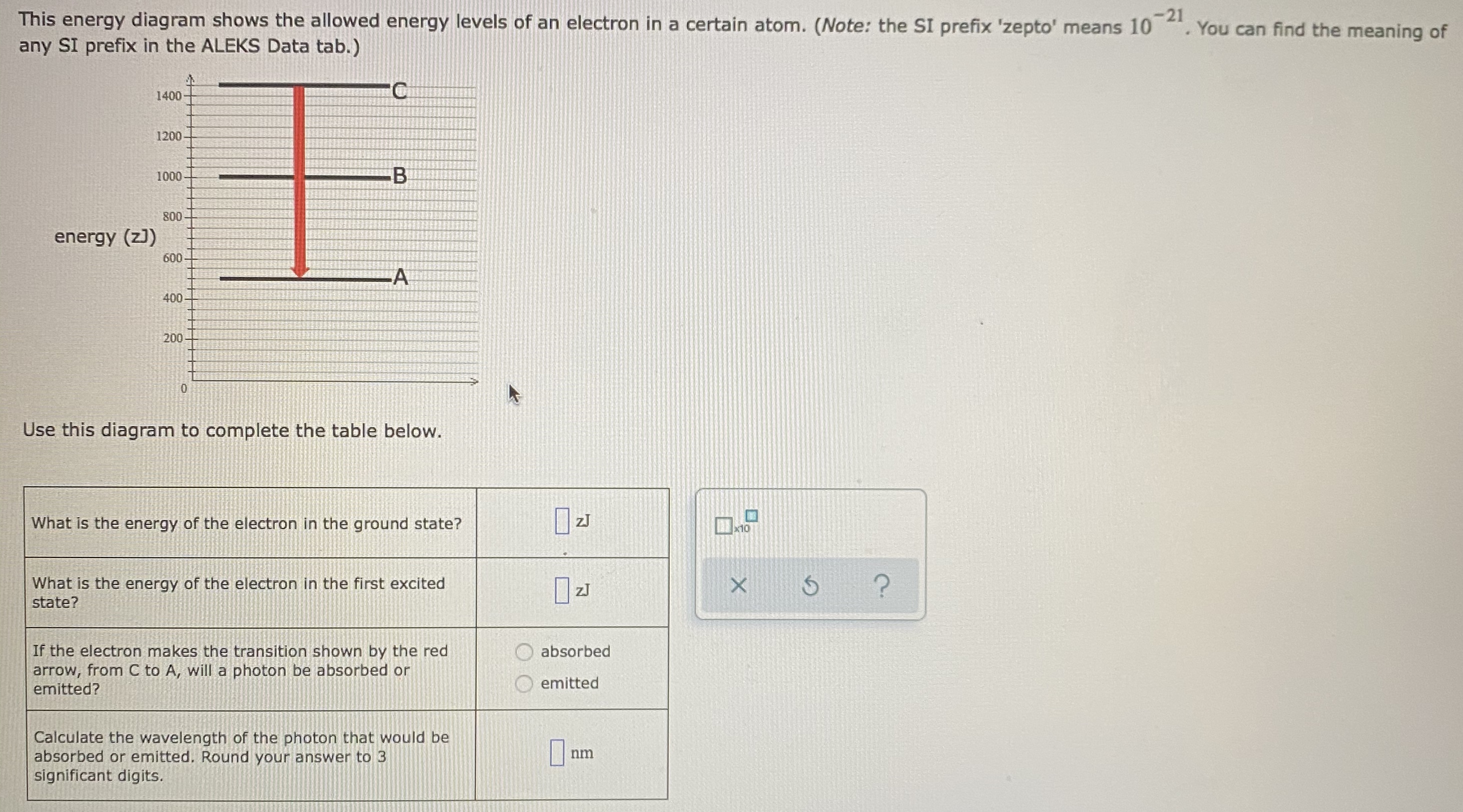
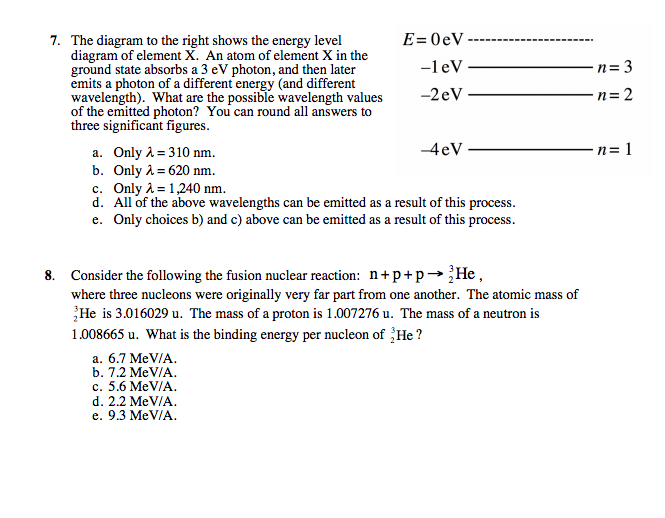











0 Response to "36 for this question consider the figure to the right shows an energy level diagram for a certain atom"
Post a Comment