40 co molecular orbital diagram
Feb 03, 2021 · A simplified molecular orbital diagram for an octahedral transition metal complex showing σ−and π−interactions only. Class I : In class I complexes, the Δ o splitting is small and often applies to 3d metals and σ ligands at lower end of the spectrochemical series. Apr 06, 2021 · Arrange the following molecular species in increasing order of stability. Answer: N 2 2-< N 2-= N 2+ < N 2. Question 48. Explain on the basis of the molecular orbital diagram why O 2 should be paramagnetic? Answer: O 2 molecule contains one unpaired electron in each of one π2p x and π2p y orbitals. Question 49. Define antibonding molecular ...
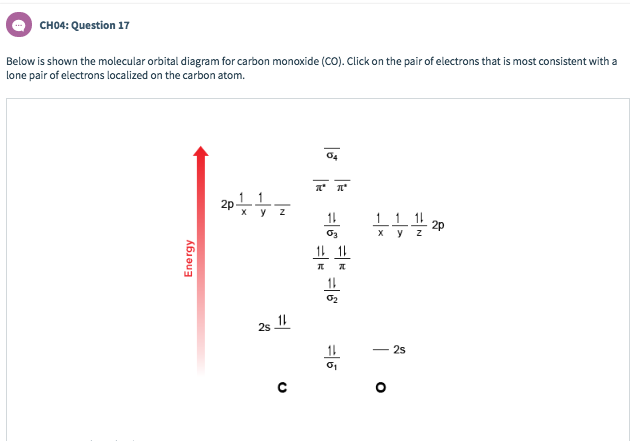
Molecular Orbital diagram of Carbon monoxide molecule (CO): ... CO =σ1s2,σ*1s2,σ2s2,σ*2s2, σ2px2, π2py2= π2pz2.

Co molecular orbital diagram
19 Mar 2021 — Carbon monoxide MO diagram ... Carbon monoxide is an example of a heteronuclear diatomic molecule where both atoms are second-row elements. The ...Molecular orbital diagrams for... · Carbon monoxide MO diagram Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules. The orbital ellipse itself precesses in space, in an irregular fashion, completing a full cycle every 112,000 years relative to the fixed stars. Apsidal precession occurs in the plane of the ecliptic and alters the orientation of the Earth's orbit relative to the ecliptic. This happens primarily as a result of interactions with Jupiter and Saturn.
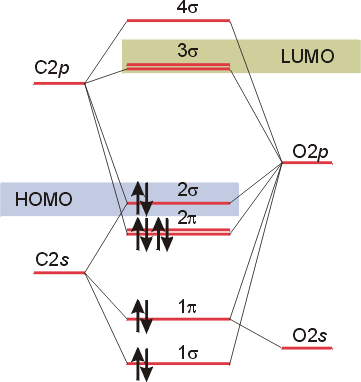
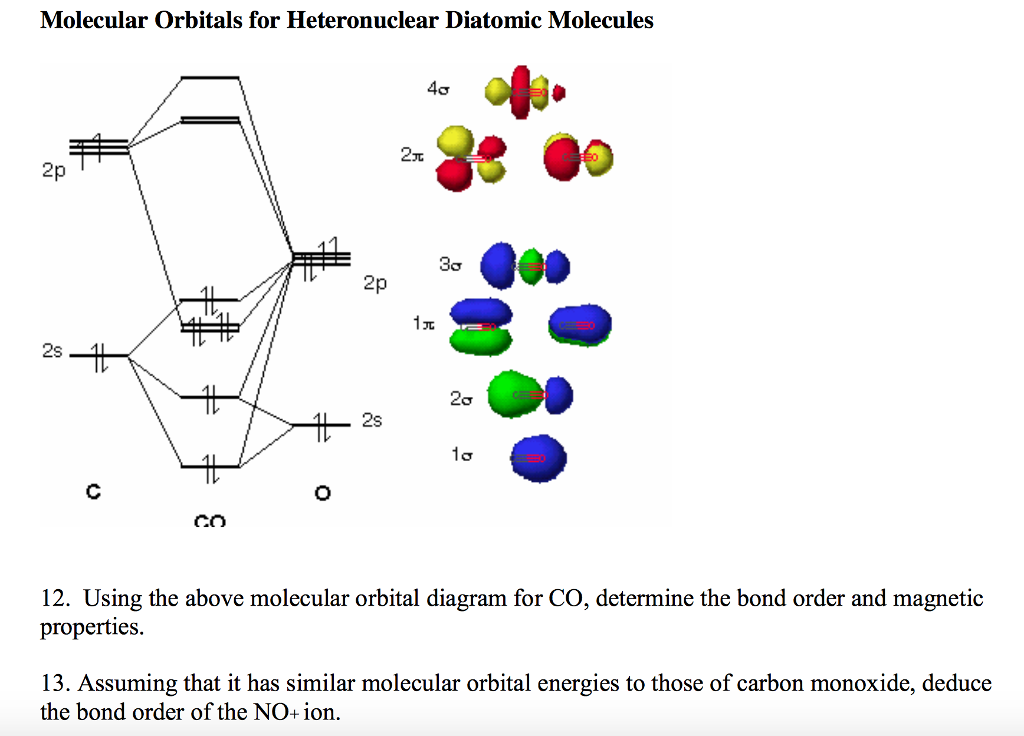
Co molecular orbital diagram. The Molecule · CO is a very stable 10-valence-electron molecule, isoelectronic with [CN]– and with N2, which has a slightly lower bond dissociation energy than ... 7 Jan 2017 — Measured CO bond length is 1.128 Å, & bond length of CO+ is 1.115 Å. · * The Bond Order in CO+ is 3.5 . · The highest occupied molecular orbital (or HOMO) is the ...4 answers · 29 votes: First let us know what molecular orbital diagram is: A molecular orbital diagram, or MO diagram, ...What is the bond order of CO? - Quora16 Sep 2016Why in the MOT diagram of CO, 2p sigma has higher energy ...8 Sep 2020When we draw an MO diagram of CO or CO2, why is ... - Quora7 Sep 2020How would you describe the CO bond in terms of molecular ...25 Aug 2017More results from www.quora.com 2 answersThe electrons in the frontier orbital(s) play a special role for the chemical reactivity. In CO, the HOMO is the 5σ orbital (ref: your diagram), ... In the following diagram, two 1s atomic orbitals combine to give a sigma (σ) bonding (low energy) molecular orbital and a second higher energy MO referred to as an antibonding orbital. The bonding MO is occupied by two electrons of opposite spin, the result being a covalent bond.
Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration. In the following diagram, two 1s atomic orbitals combine to give a sigma (σ) bonding (low energy) molecular orbital and a second higher energy MO referred to as an antibonding orbital. The bonding MO is occupied by two electrons of opposite spin, the result being a covalent bond. The molecular orbital diagram of carbon monoxide is very similar to that of molecular nitrogen. Carbon, with 4 valence electrons, and oxygen with 6 valence ... In carbon monoxide (CO, isoelectronic with dinitrogen) the oxygen 2s orbital is much lower in energy than the carbon 2s orbital and therefore the degree of ...
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine The orbital ellipse itself precesses in space, in an irregular fashion, completing a full cycle every 112,000 years relative to the fixed stars. Apsidal precession occurs in the plane of the ecliptic and alters the orientation of the Earth's orbit relative to the ecliptic. This happens primarily as a result of interactions with Jupiter and Saturn. Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules. 19 Mar 2021 — Carbon monoxide MO diagram ... Carbon monoxide is an example of a heteronuclear diatomic molecule where both atoms are second-row elements. The ...Molecular orbital diagrams for... · Carbon monoxide MO diagram

1 Draw The Molecular Orbital Diagram Of Transition Metal Ion In High Spin Mn H2o 4 Oh 2 Complex Also Determine Homeworklib













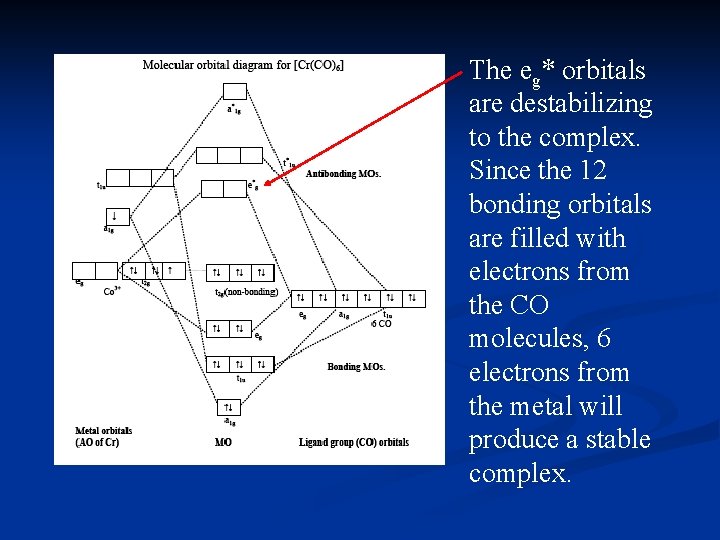




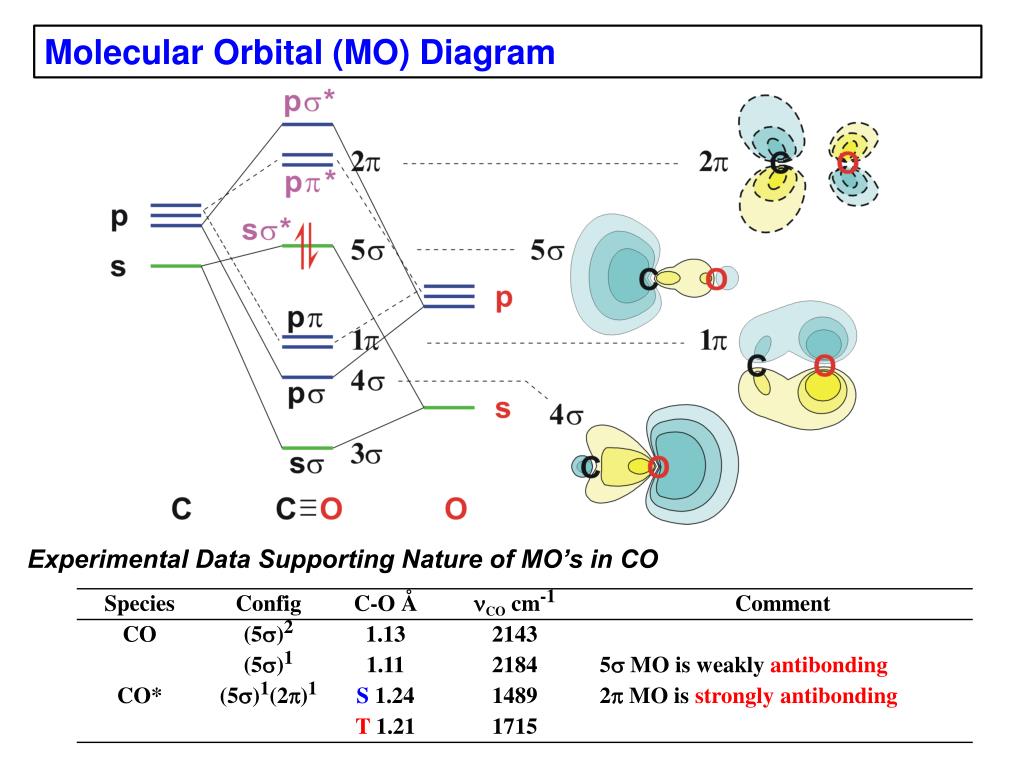



0 Response to "40 co molecular orbital diagram"
Post a Comment