39 single component phase diagram
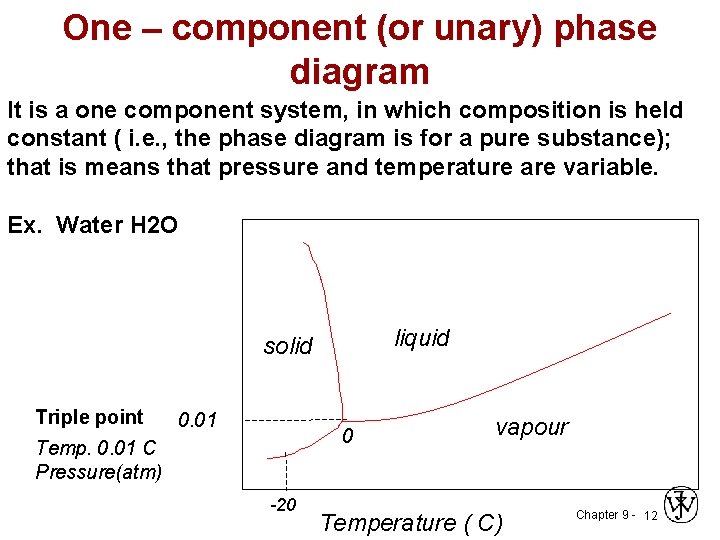
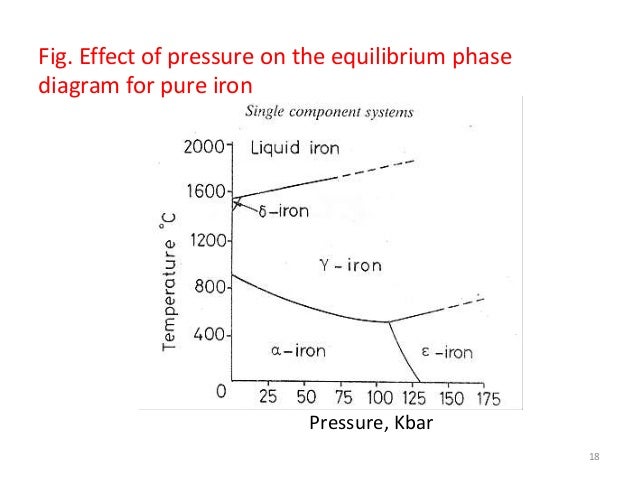
Single Component Phase Equilibria. When there is only one degree of freedom in a single component phase diagram, it was shown above that there must be a relation between and for the system to remain in two phase equilibrium. Such a relation can be derived as follows: (26-2) A single-component phase diagram can be simply a one- or two-dimensional plot, showing the phase changes in the given component (e.g., in iron) as temperature and/or pressure changes. Most diagrams, however, are two-dimensional plots describing the phase relationships in systems made up of two of more components.
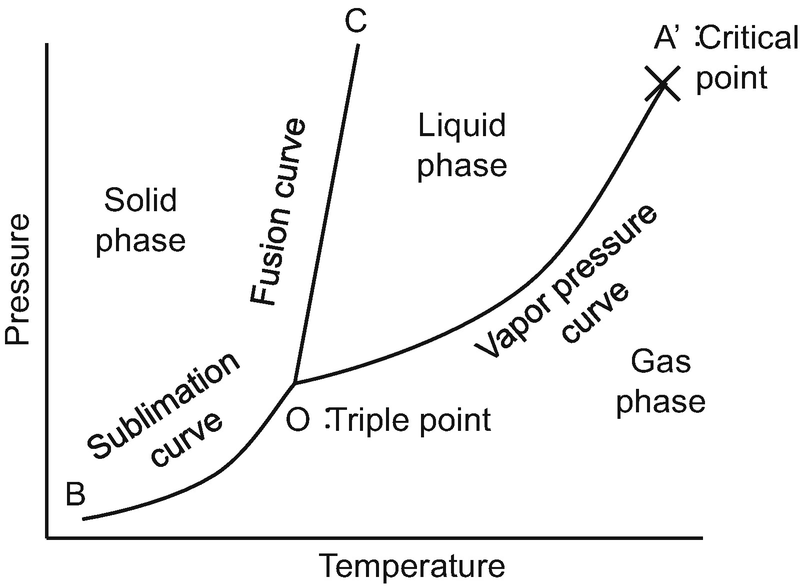
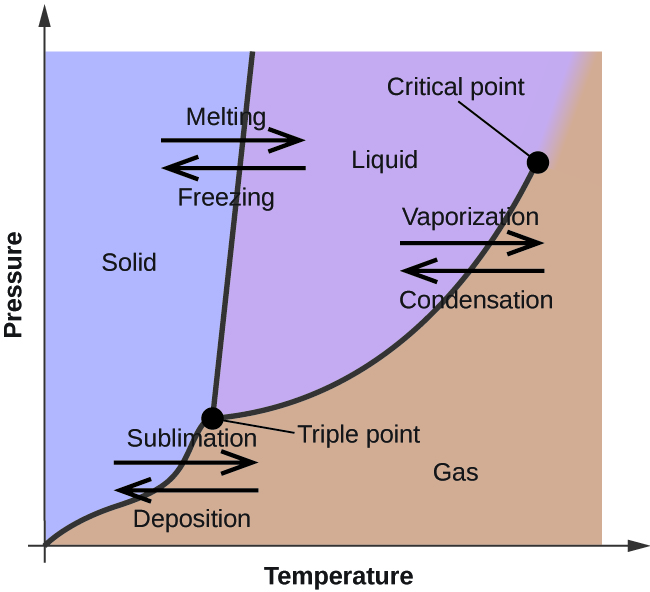
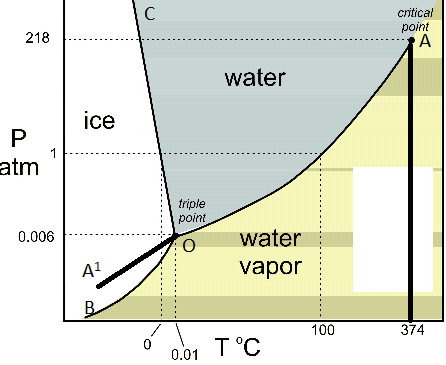
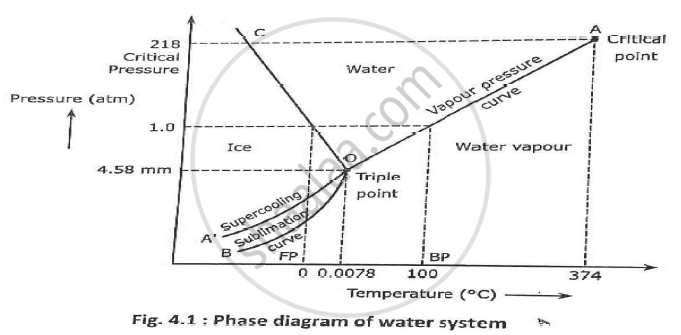
A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures ...
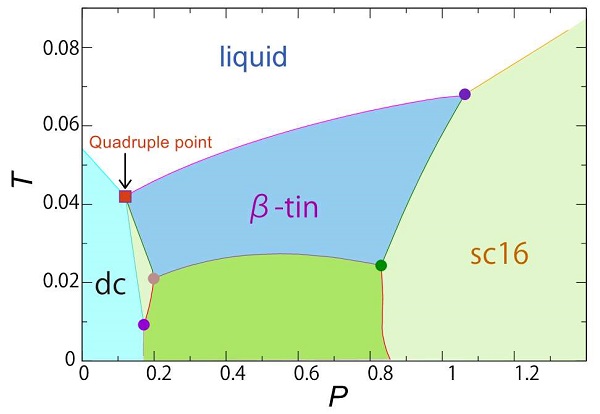
Single component phase diagram
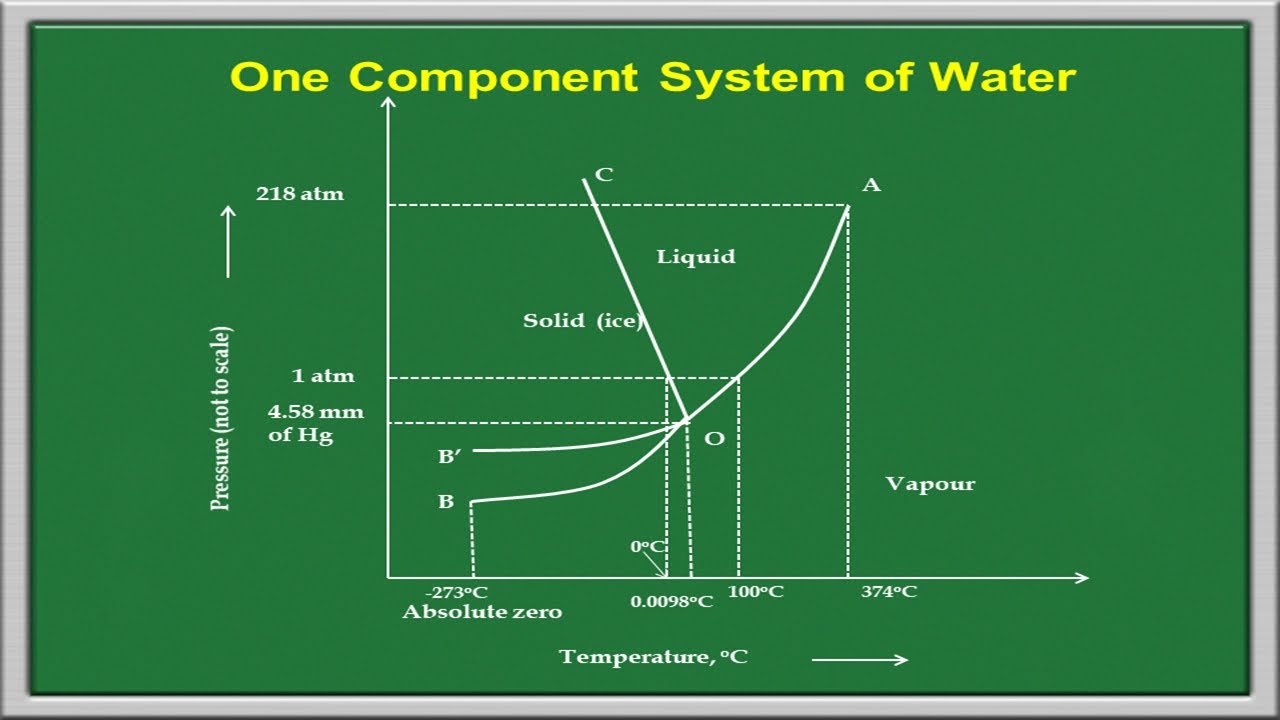
We will limit our discussion of phase diagrams of multi-component systems to binary alloys and will assume pressure to be constant at one atmosphere. Phase diagrams for materials with more than two components are complex and difficult to represent. An example of a phase diagram for a ternary alloy is shown for a fixed T and P below. Phase Diagram:One Component System 1. PHASE DIAGRAMS INTRODUCTION Engineering materials are known by their set of properties. The properties depends to a large extent on microstructure and macrostructure. Macrostructure, it can be observed with the naked eye or maybe even upto 15 times magnification. The macrostructure gives information on the material's grain size, shape and defects present ... In the phase diagram to the right, the boundary curve between the liquid and gas regions maps the constraint between temperature and pressure when the single-component system has separated into liquid and gas phases at equilibrium. The only way to increase the pressure on the two phase line is by increasing the temperature. If the temperature is decreased by cooling, some of the gas condenses ...
Single component phase diagram. Purpose: An example of implementation level UML class diagram to illustrate usage of Android Camera API (Android 3.1 Platform, API Level 12). Summary: CameraDemo class extends Android's Activity class. An Activity is a single, focused thing that a user can do with Android. Activity usually interacts with user, and the Activity class takes care ... Single Component Phase Diagrams For a single component an equation of state relates the variables of the system, PVT PV = RT or Z = 1 Ideal Gas at low p or high T or low r Virial Equation of State So a phase diagram will involve two free variables, such as P vs T or T versus r. Example - Gibbs phase rule in eutectic diagram In the double phase regions: N = 1 (the diagram is plotted for fixed pressure, 1 atm) C = 2 P = 2 F = N + C -P F = 1 By fixing the Temp one determines the compositions in the liquid and in the solid i.e. for certain Temp there is a fixed compositions In the single phase regions: N = 1 (the diagram ... Iron-Carbon Phase Diagram. The iron-carbon phase diagram is widely used to understand the different phases of steel and cast iron. Both steel and cast iron are a mix of iron and carbon. Also, both alloys contain a small amount of trace elements. The graph is quite complex but since we are limiting our exploration to Fe3C, we will only be ...
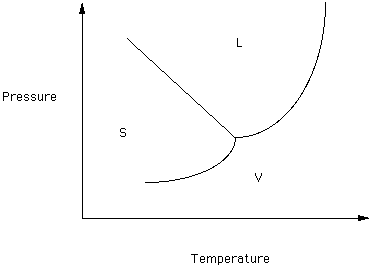
Phase diagrams can be used to understand the range of thermodynamic variables over which a pure sample of matter exists as a particular state/phase. Phase diagrams are divided into three single phase regions that cover the pressure - temperature space over which the matter being evaluated exists: liquid, gaseous, and solid states. 2D phase diagram. A phase diagram. The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water. The axes correspond to the pressure and temperature. The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas. 3.012 Fundamentals of Materials Science Fall 2005 Phases and phase diagrams single-component materials • MAIN IDEA: Single-component phase diagrams map out in P-T, P-V, or P-V-T space the identities of the stable equilibrium form or forms of a material as a function of the environmental conditions. 12.02.2021 · COMPLETE CIRCUIT DIAGRAM ... Please I need to measure three phase voltage line to line and three. Phase current by using pic18f4550. Reply. prabakaran. October 12, 2014 at 5:55 pm good tutorial can u send me the simulation. Reply. BILAL Malik. October 12, 2014 at 5:58 pm Use pictures given in article to draw circuit diagram. Reply. Abuzur. October 27, 2014 at 12:19 pm Dear Bilal, Good …
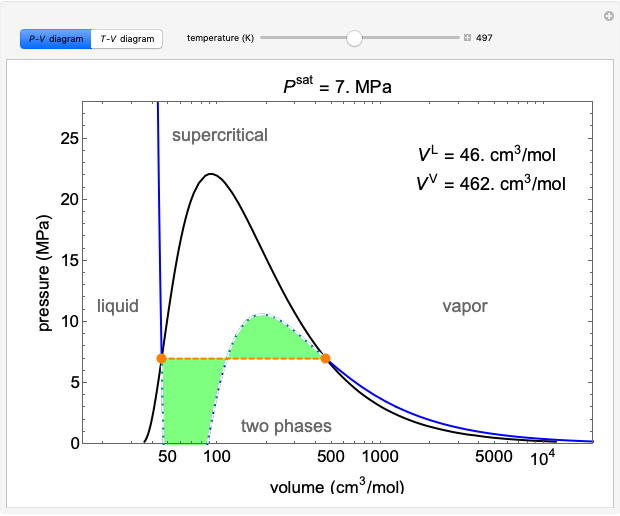
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... The phase diagrams (P-V, P-T, V-T) typically used to represent single-component phase behavior are 2-D projections of a 3-D surface. A liquid can be converted to a vapor without the co-existence of two phases by circumnavigating the critical point. At a given pressure, vapor and liquid are in equilibrium at only one temperature. 1.2 One-Component Phase Diagrams and Gibbs Phase Rule 11:04. 1.3 Regions of Two Phase Equilibrium 8:53. 1.4 Additional One-Component Phase Diagrams 3:27. Taught By. Thomas H. Sanders, Jr. Regents' Professor. Try the Course for Free. Transcript. Explore our Catalog Join for free and get personalized recommendations, updates and offers. ... Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
Fig. 8.3 is a pressure-composition (p-x-y) phase diagram that shows typical vapor/liquid phase behavior for a binary system at a fixed temperature below the critical temperature of both components. At pressures below the vapor pressure of Component 2, p v2, any mixture of the two components forms a single vapor phase.At pressures between p v1 and p v2, two phases can coexist for some compositions.
Determine the state of a single-component system at a given pressure and temperature, given a phase diagram. Explain how a single-component system changes as temperature or pressure changes. Explain the significance of a critical point and identify it on a single-component phase diagram.
Briefly describes the various diagrams used to represent single-component phase equilibrium. The diagrams shown include pressure-temperature, pressure-volume...
The phase diagrams (P-V, P-T, V-T) typically used to represent single-component phase behavior are 2-D projections of a 3-D surface. A liquid can be converted to a vapor without the co-existence of two phases by circumnavigating the critical point. At a given pressure, vapor and liquid are in equilibrium at only one temperature.
Phase diagrams Clapeyron Equation Phase Rule • Phase Equilibria in a One Component System Goal: Understand the general phenomenology of phase transitions and phase coexistence conditions for a single component system. The Chemical Potential µ controls phase transitions and phase equilibria, as well as equilibrium in chemical reactions.
A single- component phase diagram can be simply a one- or two-dimensional plot showing the phase changes in the substance as temperature and/or pressure change. Most diagrams, however, are two- or three-dimensional plots describing the phase relationships in systems made up of two or more components, and these usually contain ...
TWO COMPONENT EUTECTIC SYSTEMS. Figure 1 shows the simplest of two component phase diagrams. The components are A and B, and the possible phases are pure crystals of A, pure crystals of B, and liquid with compositions ranging between pure A and pure B. Compositions are plotted across the bottom of the diagram.
This condition dictates that, for a single-component system, the maximum number of phases that can coexist is three. Historically this is known as the Gibbs phase rule, and is one of the oldest ...
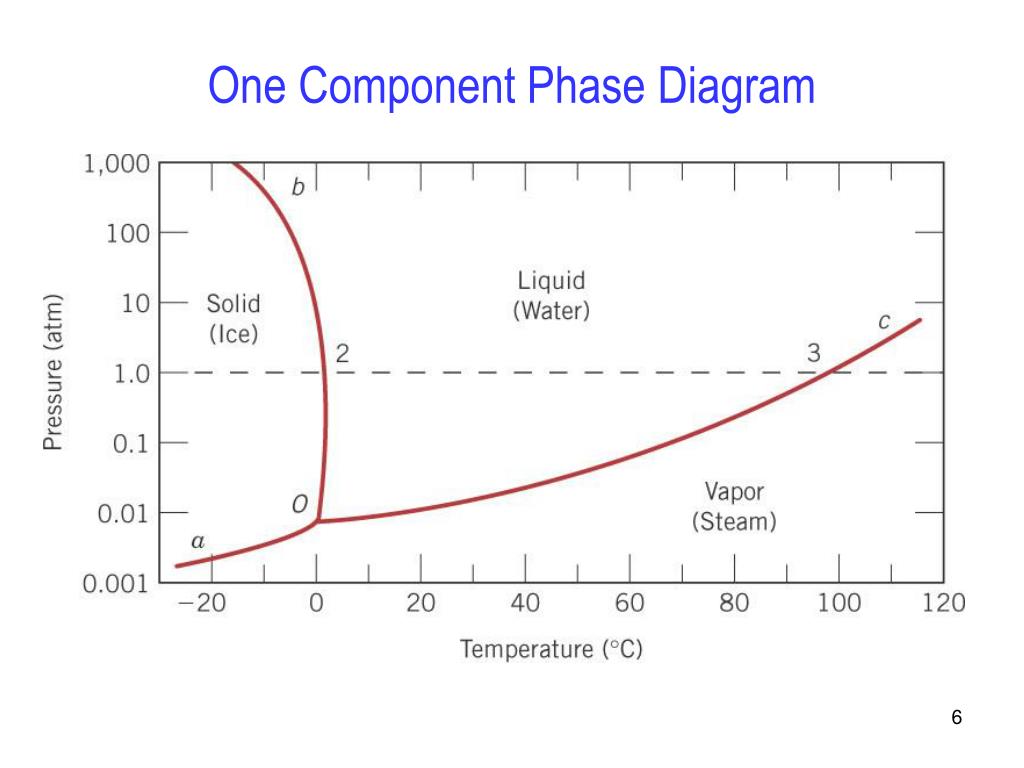
8.2: Single Component Phase Diagrams. The stability of phases can be predicted by the chemical potential, in that the most stable form of the substance will have the minimum chemical potential at the given temperature and pressure. This can be summarized in a phase diagram like the one shown below. In this diagram, the phase boundaries can be ...
If we were to plot the phase state (number and types of phases) on a pressure-temperature plot for this single-component system, then we would obtain a plot like that shown in Figure 2.09. This figure is also referred to as a Phase Diagram or a P-T Diagram.In this Phase Diagram, the grey region represents all of the pressure-temperature combinations that result in the solid form of our single ...
Figure 13.1: The Pressure-Composition Phase Diagram of an Ideal Solution Containing a Single Volatile Component at Constant Temperature. As emerges from Figure 13.1 , Raoult's law divides the diagram into two distinct areas, each with three degrees of freedom. 57 Each area contains a phase, with the vapor at the bottom (low pressure), and ...
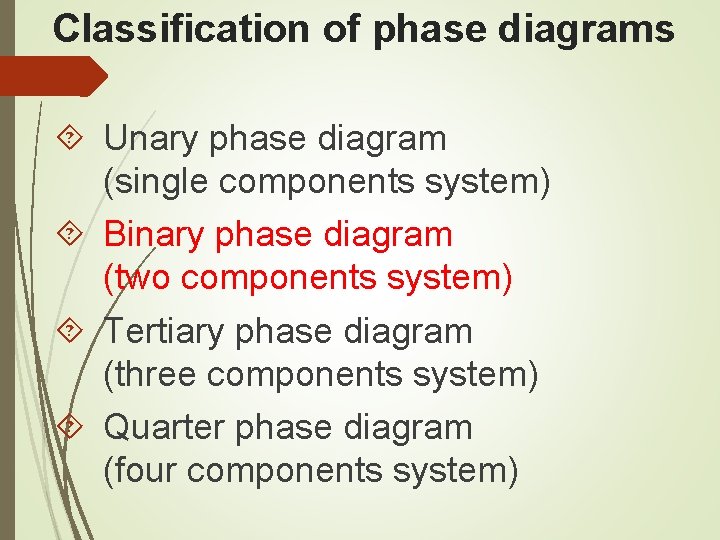
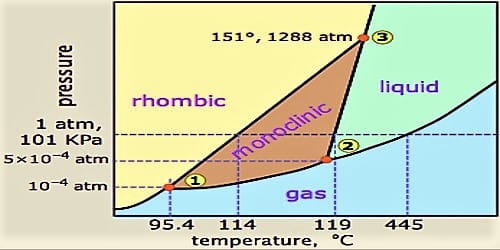
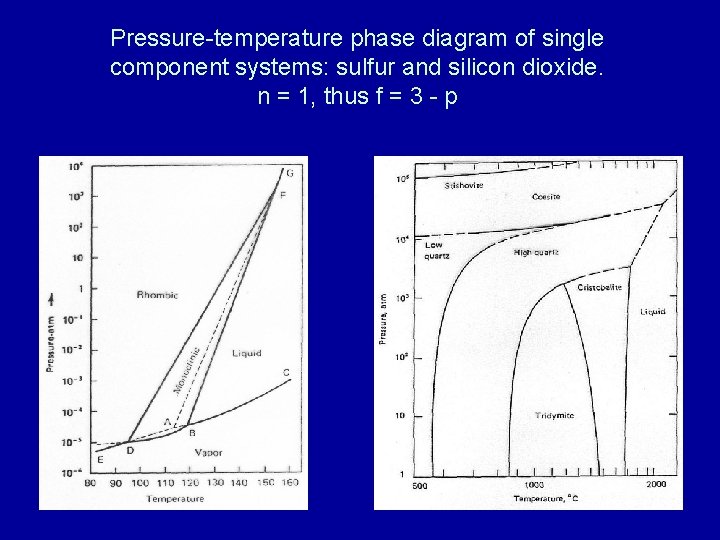
Unary Phase Diagram (Single Component): It is used mainly for carbon and pure metal. There is very limited practical utilities of such diagram plotted between temperature and pressure axis. Example: ADVERTISEMENTS: Water, graphite, metallic carbon, diamond. 2. Binary Phase Diagram (Two Components):
The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water.The axes correspond to the pressure and temperature.The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas.. The curves on the phase diagram show the points where the free energy (and other ...
In the phase diagram to the right, the boundary curve between the liquid and gas regions maps the constraint between temperature and pressure when the single-component system has separated into liquid and gas phases at equilibrium. The only way to increase the pressure on the two phase line is by increasing the temperature. If the temperature is decreased by cooling, some of the gas condenses ...
Phase Diagram:One Component System 1. PHASE DIAGRAMS INTRODUCTION Engineering materials are known by their set of properties. The properties depends to a large extent on microstructure and macrostructure. Macrostructure, it can be observed with the naked eye or maybe even upto 15 times magnification. The macrostructure gives information on the material's grain size, shape and defects present ...
We will limit our discussion of phase diagrams of multi-component systems to binary alloys and will assume pressure to be constant at one atmosphere. Phase diagrams for materials with more than two components are complex and difficult to represent. An example of a phase diagram for a ternary alloy is shown for a fixed T and P below.













0 Response to "39 single component phase diagram"
Post a Comment