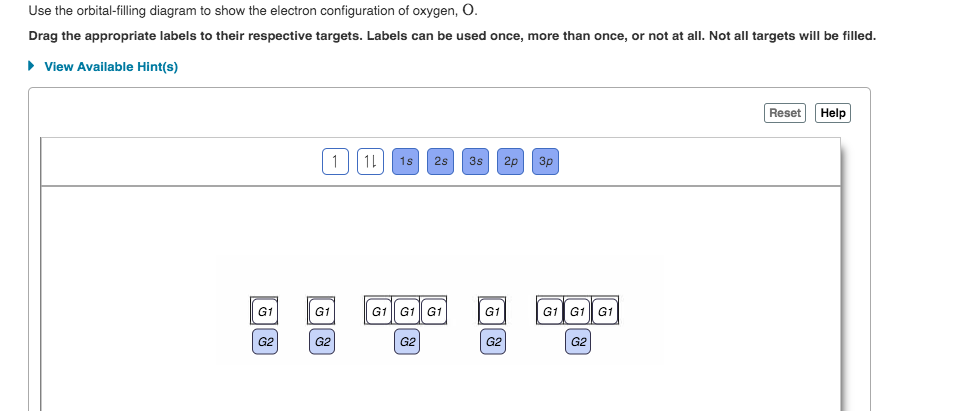
40 orbital filling diagram for oxygen
How to Do Orbital Diagrams | Sciencing Orbital diagrams give you all of the information you need about the electron configuration and occupied spin states for chemistry or physics, and See Resources for a diagram showing the filling order. Note that the n = 1 level only has s orbitals, the n = 2 level only has s and p orbitals, and the n... Orbital-filling diagram - HomeworkLib Show the orbital-filling diagram for (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshellat 13. In polyatomic atoms electrons fill the orbitals from the lowest energy to the highest. Write the electron configuration of oxygen.
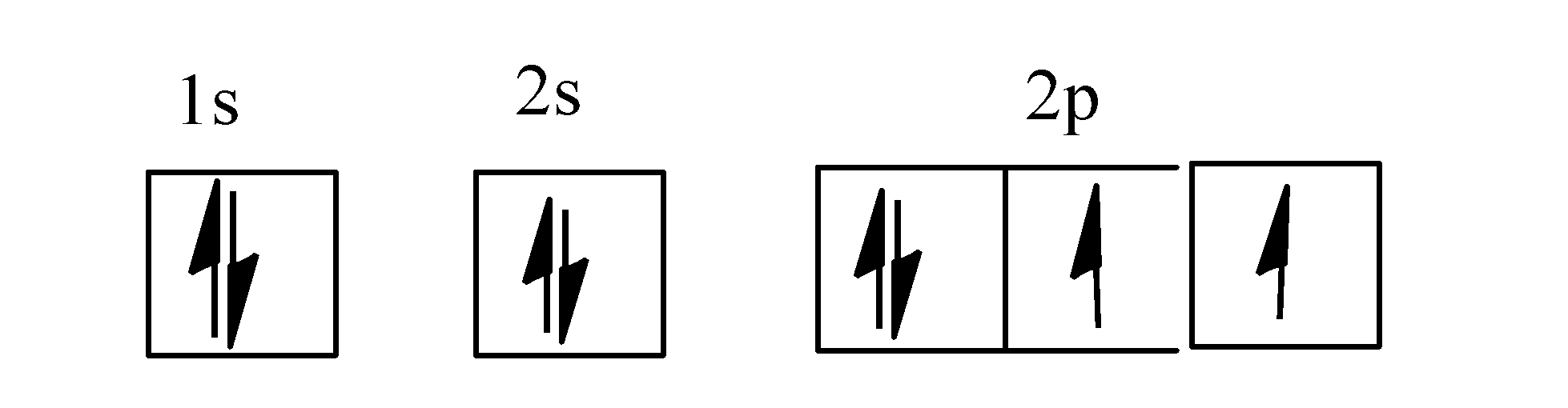
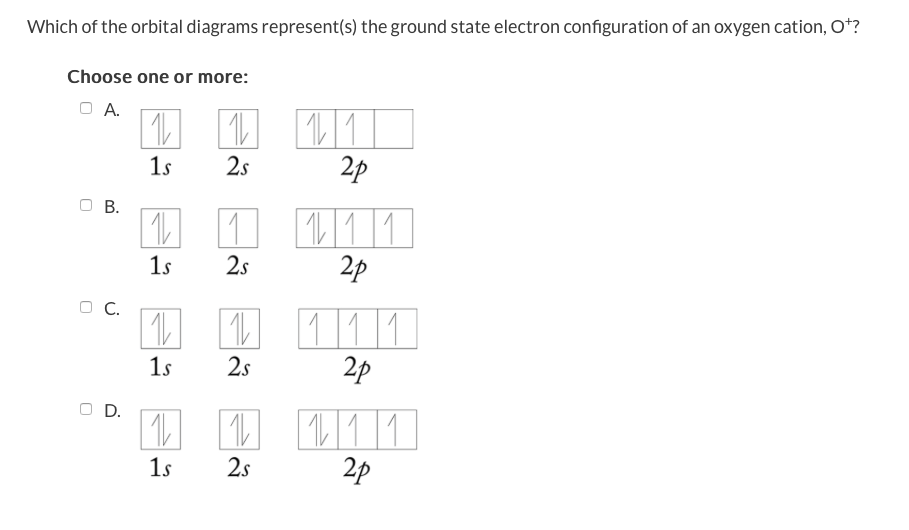
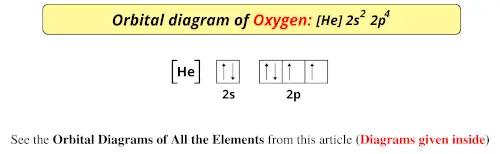
Electron Configurations - Course Hero In the orbital box diagram for oxygen, the electrons in the 2p degenerate orbital fill out as singletons before doubling up with an electron of opposite spin. According to Hund's rule, when filling orbitals of the same energy, electrons occupy empty orbitals before doubly occupying the same orbital.
Orbital filling diagram for oxygen
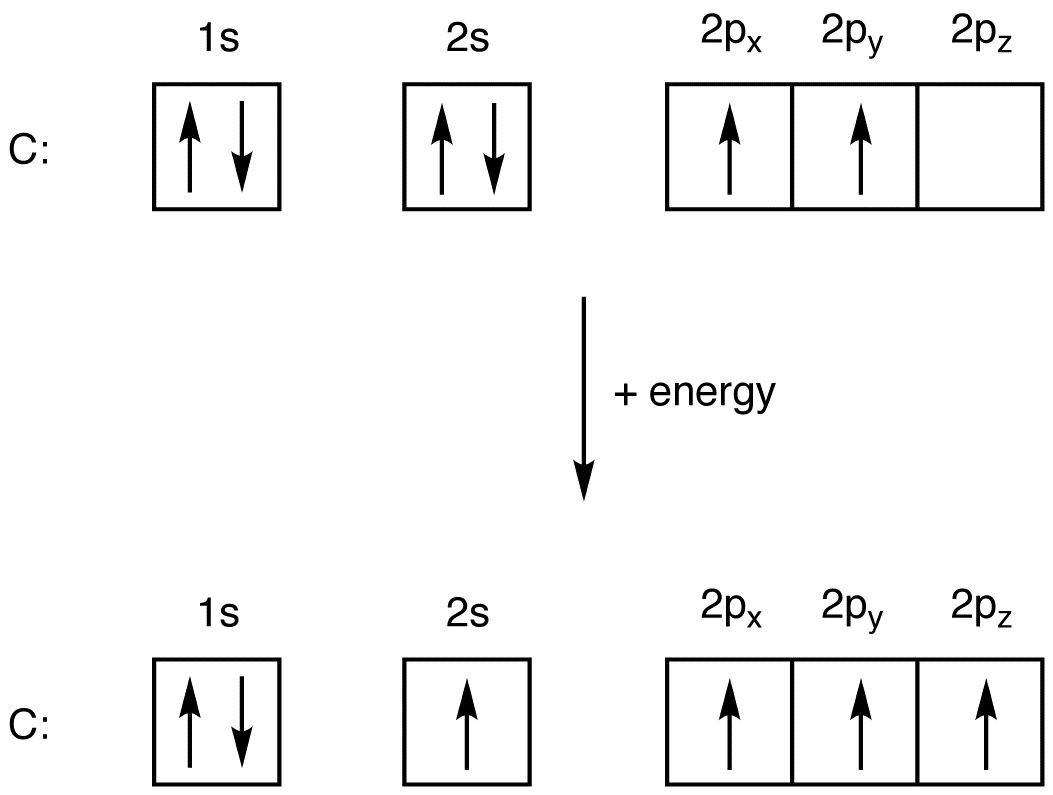
Orbital Filling Diagram For Nitrogen - Drivenhelios Show the orbital filling diagram for rm n nitrogen best answer the electronic configuration for nitrogen atom is 1s 2 2s 2 2p 3 lowest energy state will What Is The Molecular Orbital Diagram For Oxygen Quora. Electron Configurations The Periodic Table. 12 9 Orbital Shapes And Energies Chemistry... Oxygen(O) electron configuration and orbital diagram To create an orbital diagram of an atom, you first need to know Hund's principle and Pauli's exclusion principle. Hund's principle is that electrons in different orbitals with How can oxygen become a stable electron configuration? Ans: The oxygen atom takes 2 electrons to fill the octave and become stable. Chapter 5 Flashcards | Quizlet Using the orbital filling diagram for oxygen, explain how it obeys Aufbau Principle, Pauli Exclusion Principle and Hund's Rule. Aufbau: Fill the 1s, then the 2s, then the 2p Pauli: 2 e- in the same orbital must have opposite spins Hund's: In the 2p sublevel, the first 3 e- are placed in different orbitals in the...
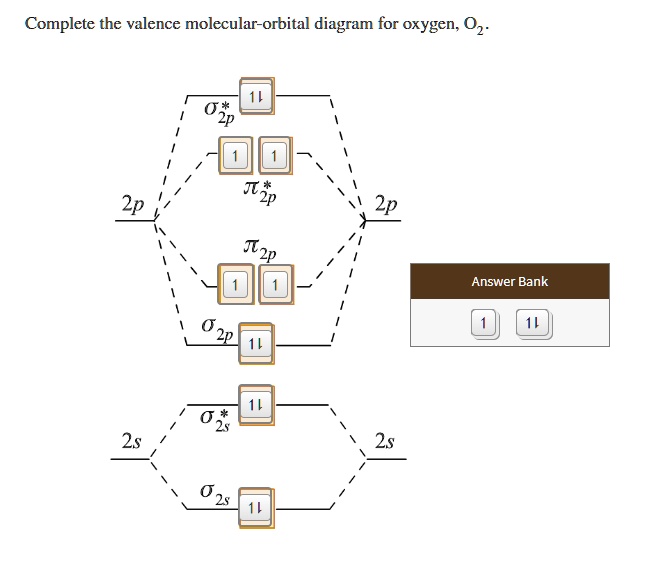
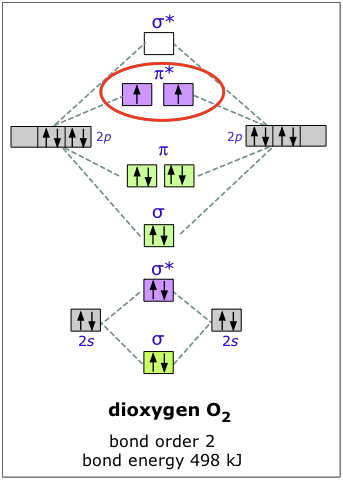
Orbital filling diagram for oxygen. How do yo write the orbital diagram for oxygen? | Socratic The electron configuration for oxygen is: 1s^2 2s^2 2p^4 This video will walk you through the step of writing orbital diagram. The video uses Kr as an example, but the process is exactly as the same as what you need to do for oxygen. Hope this helps! Tutorial on Chemical Bonding, Part 8 of 10 (Molecular orbitals) The diagram shows how the molecular orbitals in lithium hydride can be related to the atomic However, the order in which these two orbitals are filled has no effect on the predicted bond orders, so Since molecular oxygen contains two electrons in an antibonding orbital, it might be possible to... Molecular Orbital diagram for the molecule, oxygen, O2. - YouTube This video shows the construction of a molecular orbital (MO) diagram for the diatomic molecule, O2, using the valence electrons of each oxygen. Orbital Diagrams & Electron Configurations for Atoms and Ions Orbital filling order for elements beyond Period 2 … ...corresponds to atom's location in periodic table! Draw an orbital diagram for beryllium (Z=4) 1s Guidelines for drawing 1s 2s 7 N nitrogen 14.01 2p Ti 8 O oxygen 16.00 Orbital diagrams for ions • anion (negative charge): ADD appropriate number...
Example 1: Write the orbital filling diagram of oxygen - StuDocu Orbital filling diagrams represent the electrons as arrows assigned to their sublevel orbitals. 1. Write the complete orbital filling diagrams for the following Chapter 5 Orbital Filling Diagrams and Electron Dot Diagrams. 7 Orbital Filling Diagrams. 8 A. General Rules Pauli Exclusion Principle -Each orbital can hold TWO electrons with opposite spins. 11 What type of sublevel is this? 12 Make the orbital filling diagram for d 8 How many of the electrons are unpaired? 18 Draw the electron dot diagram for oxygen. Chapter 5 Flashcards | Quizlet Using the orbital filling diagram for oxygen, explain how it obeys Aufbau Principle, Pauli Exclusion Principle and Hund's Rule. Aufbau: Fill the 1s, then the 2s, then the 2p Pauli: 2 e- in the same orbital must have opposite spins Hund's: In the 2p sublevel, the first 3 e- are placed in different orbitals in the... Oxygen(O) electron configuration and orbital diagram To create an orbital diagram of an atom, you first need to know Hund's principle and Pauli's exclusion principle. Hund's principle is that electrons in different orbitals with How can oxygen become a stable electron configuration? Ans: The oxygen atom takes 2 electrons to fill the octave and become stable.
Orbital Filling Diagram For Nitrogen - Drivenhelios Show the orbital filling diagram for rm n nitrogen best answer the electronic configuration for nitrogen atom is 1s 2 2s 2 2p 3 lowest energy state will What Is The Molecular Orbital Diagram For Oxygen Quora. Electron Configurations The Periodic Table. 12 9 Orbital Shapes And Energies Chemistry...





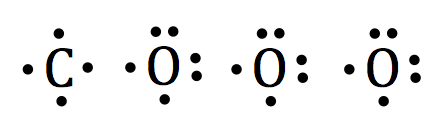




















0 Response to "40 orbital filling diagram for oxygen"
Post a Comment