38 diagram of salt dissolving in water
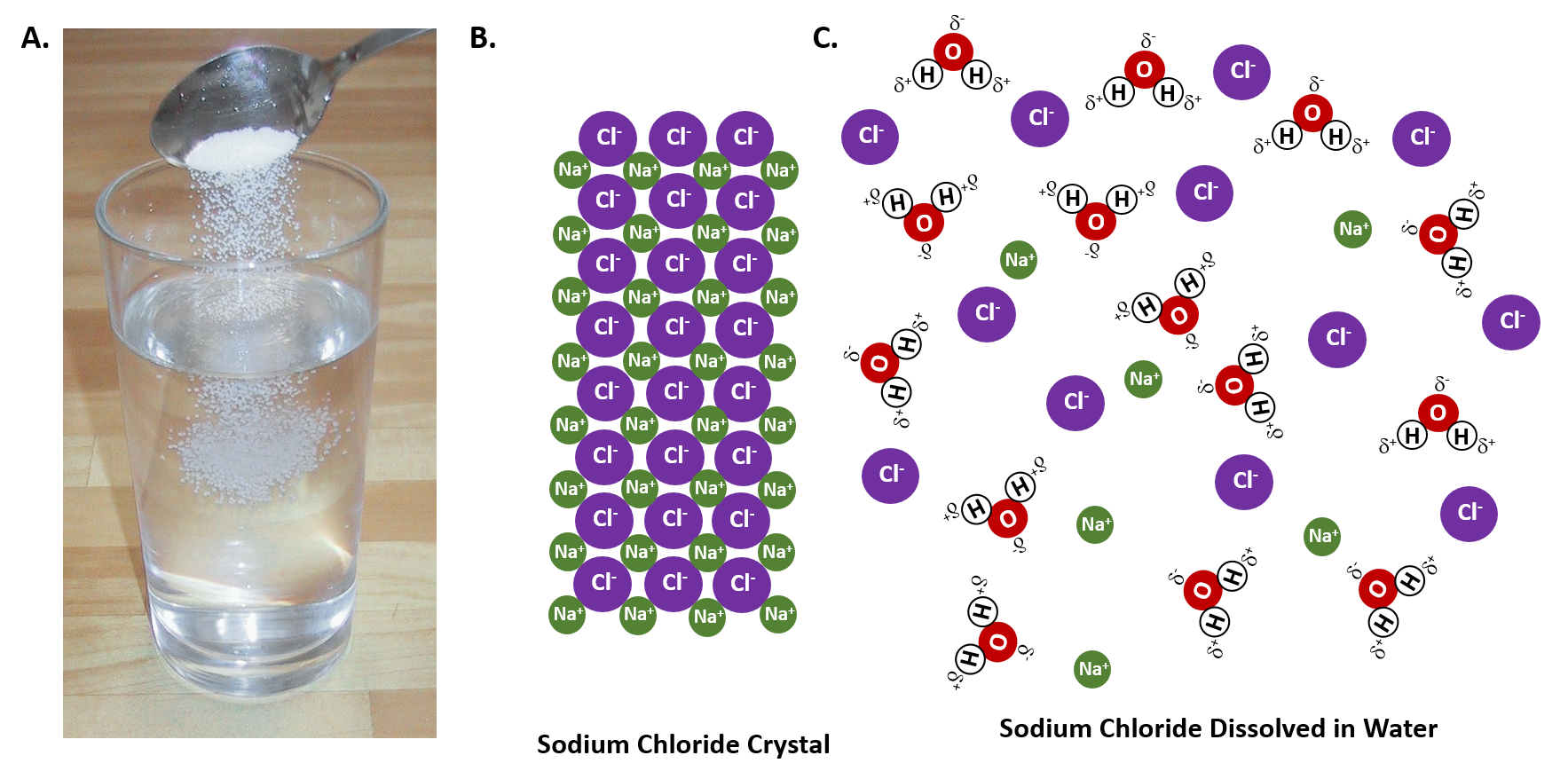
The diagram shows how salt is removed from sea water to ... The diagram shows different stages in the removal of salt from seawater, in order to make water much pure and drinkable. First of all, salty water is taken by pre-treatment filter from the sea. The water therefore is forced through membrane at high temperature. As a result, impurities and salt are removed from water molecules. Dissolving Salt in Water: Chemical or Physical Change? When you dissolve salt in water, the sodium chloride dissociates in Na + ions and Cl - ions, which may be written as a chemical equation : NaCl (s) → Na + (aq) + Cl - (aq) Therefore, dissolving salt in water is a chemical change. The reactant (sodium chloride, or NaCl) is different from the products (sodium cation and chlorine anion).
Dissolving - Physical changes - KS3 Physics Revision - BBC ... Conservation of mass The solute does not cease to exist when it dissolves. If the water in the solution is evaporated, the solute is left behind. The total mass stays the same during dissolving....
Diagram of salt dissolving in water
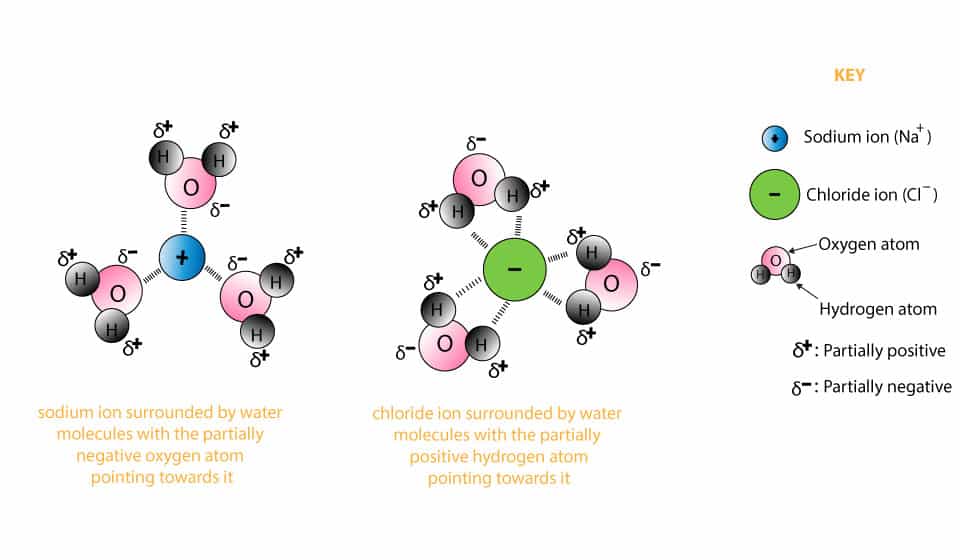
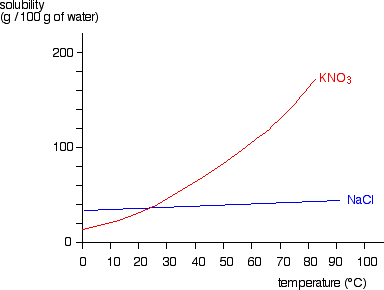
Dissolving and Back Again - American Chemical Society Place 1 teaspoon of salt into a clear plastic cup. Add about 20 milliliters (4 teaspoons) of water to the cup with the salt and swirl until most or all the salt dissolves. Pour the salt water into a shallow container like a petri dish or yogurt container lid and set aside for 24 hours. Ask students: Solid-liquid Phase Diagrams: Salt Solution SOLID-LIQUID PHASE DIAGRAMS: SALT SOLUTION. This page looks at the phase diagram for mixtures of salt and water - how the diagram is built up, and how to interpret it. It includes a brief discussion of solubility curves. Important: This page is only really designed to be an introduction to the topic suitable for courses for 16 -18 year olds ... Water, the Universal Solvent | U.S. Geological Survey Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
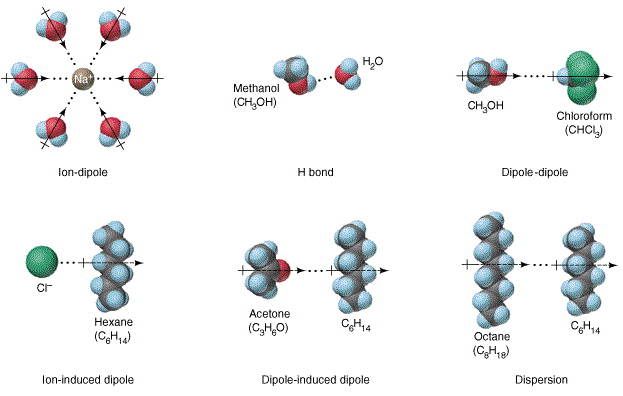
Diagram of salt dissolving in water. How Does Salt Dissolve in Water? - Reference.com Water dissolves salt by dissociating the ions in salt from each other. Because water is a polar molecule, each of its ends holds a slight positive or negative electrical charge. These ends attract the positive and negative ions in salt and pull them apart from each other. The polarity of water comes from the differences in electronegativity in ... Solved A) Draw two molecules of MgCl2 dissolved in water ... A) Draw two molecules of MgCl2 dissolved in water (show at least 6 water molecules and their interactions with each other and with the salt ions). (HINT: MgCl2 is a salt and made with ionic bonds). In your diagram, label at least one hydrogen and one polar-covalent bond. Dissolving - BBC Bitesize Salt is insoluble in propanone. Polystyrene is insoluble in water, but soluble in propanone. When an insoluble white powder is stirred into a colourless liquid it does not dissolve. At first the... The diagram below shows how salt is removed from sea water ... The diagram demonstrates the process of desalination and how the water from sea can turn into the suitable water that people can drink. First of all, the saltwater is taken from the sea and passed through the pre-treatment filter which at this stage big impurities are removed. Besides, the filter backwash is piped into the sea.
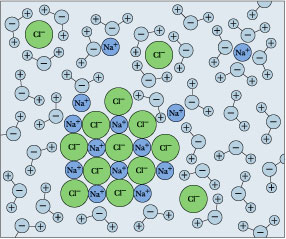
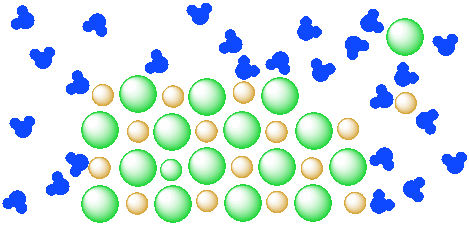
DIAGRAM >> A representation of the dissolving of sodium ... DIAGRAM >> A representation of the dissolving of sodium molecules in water The negative ends of the polar water molecules are attracted to the positive sodium ions, and pull them off the crystal lattice. The positive ends of the water molecules are attracted to the negative chloride ions, and pull them off. Draw a diagram of table salt (NaCl) dissolved in water ... Find step-by-step Biology solutions and your answer to the following textbook question: Draw a diagram of table salt (NaCl) dissolved in water.. SOLVED:Draw a diagram of table salt (NaCl) dissolved in water. we can easily draw a diagram of sodium chloride dissolved in water. Let's just do some quick review, though. Sodium chloride is in a C l. And remember, sodium has a positive charge because it has one extra proton in the nucleus than electrons in the outer cloud. What Happens When Salt Is Added to Water? - Sciencing Solids dissolving in a liquid solvent (usually water in chemistry experiments) create a solution, and salt dissolving in water is a classic example of how a polar solute behaves in a polar solvent such as H 2 O. Along the way, you'll get a side dish of acid-base chemistry just to round out the "flavor" of the salt-water experience!
FAQ: What kind of change occurs when salt dissolves in water? What happens when salt dissolves in water? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution. Water molecules and their interaction with salt | U.S ... This diagram shows the positive and negative parts of a water molecule. It also depicts how a charge, such as on an ion (Na or Cl, for example) can interact with a water molecule. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative ... 44 diagram of salt dissolved in water - Wiring Diagram Source Diagram of salt dissolved in water Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution. How does sodium chloride (NaCl) dissolve in water? Sodium chloride (NaCl) dissolves when water molecules continuously attack the NaCl crystal, pulling away the individual sodium (Na +) and chloride (Cl -) ions. This nonstop attack continuous until the whole NaCl crystal disintegrates. To understand this process at the molecular level, we must apply the three steps we previously discussed.
⚗️The diagram shows salt dissolved in water. What does it ... The diagram shows salt dissolved in water. What does it show about water molecules - 20635482 simonenekia2000 simonenekia2000 01/14/2021 Chemistry College answered The diagram shows salt dissolved in water. What does it show about water molecules and chloride ions? + Chloride Oxygen Hydrogen 4 Sodium 1
How Water Dissolves Salt - YouTube Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then dissolving the salt. ... Water molecules pulling apart the ions (sodium and chloride) in a salt crystal ...
Why does the volume of a solution not change when salt is ... Answer (1 of 4): Actually, the volume decreases instead of increase (that is the intuitive result) and, makes your water super cold. LOL, right? How it works. NaCl (salt), when dissolved in water, breaks apart into two ions that I won´t write here because since I can´t format properly, they will...
How to Dissolve Salt in Water: 9 Steps (with Pictures ... To dissolve salt into water, just stir it in with a spoon or spatula until you can't see the salt anymore. The higher the temperature of the water, the more salt you'll be able to dissolve, so if you want to dissolve more salt, heat up your water. You can also dissolve more salt in distilled water, since there are less contaminants.
Water, the Universal Solvent | U.S. Geological Survey Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
Solid-liquid Phase Diagrams: Salt Solution SOLID-LIQUID PHASE DIAGRAMS: SALT SOLUTION. This page looks at the phase diagram for mixtures of salt and water - how the diagram is built up, and how to interpret it. It includes a brief discussion of solubility curves. Important: This page is only really designed to be an introduction to the topic suitable for courses for 16 -18 year olds ...
Dissolving and Back Again - American Chemical Society Place 1 teaspoon of salt into a clear plastic cup. Add about 20 milliliters (4 teaspoons) of water to the cup with the salt and swirl until most or all the salt dissolves. Pour the salt water into a shallow container like a petri dish or yogurt container lid and set aside for 24 hours. Ask students:












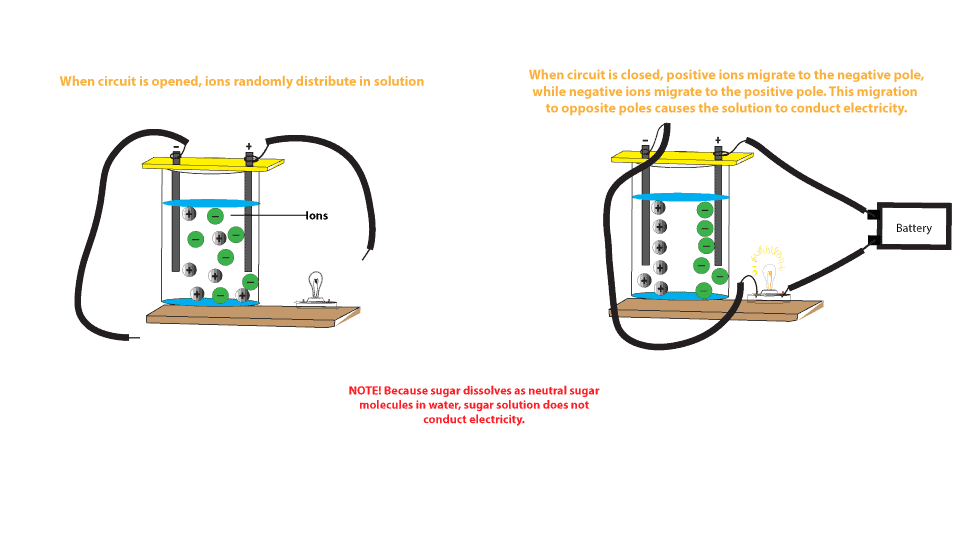




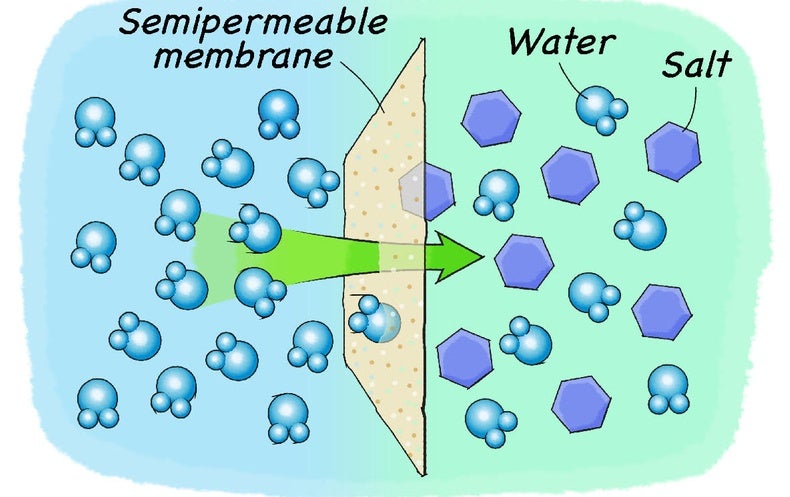



0 Response to "38 diagram of salt dissolving in water"
Post a Comment