36 molecular orbital diagram hf
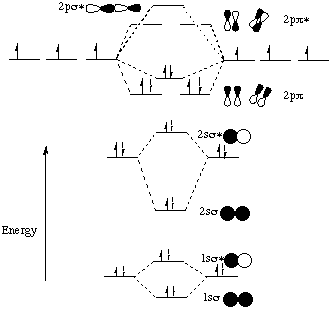
Ch2 MO Theory - DocShare.tips Molecular Orbitals for Heteronuclear Diatomic Molecules The most striking molecular orbital difference between a heteronuclear diatomic, such as HF, and the homonuclear diatomics is that the orbitals are no longer have equally density on each atom. Note that the "1-sigma" and "2-sigma" molecular orbitals of Hydrogen Fluoride are Heteronuclear Molecular orbital diagram - Summarized by ... The HF electron configuration shows that the other electrons are still in three lone pairs and that the bond order is one. While MOs for homonuclear diatomic molecules have equal contributions from each interacting atomic orbital, MOs for heteronuclear diatomic molecules differ in terms of their respective atomic orbital contributions.
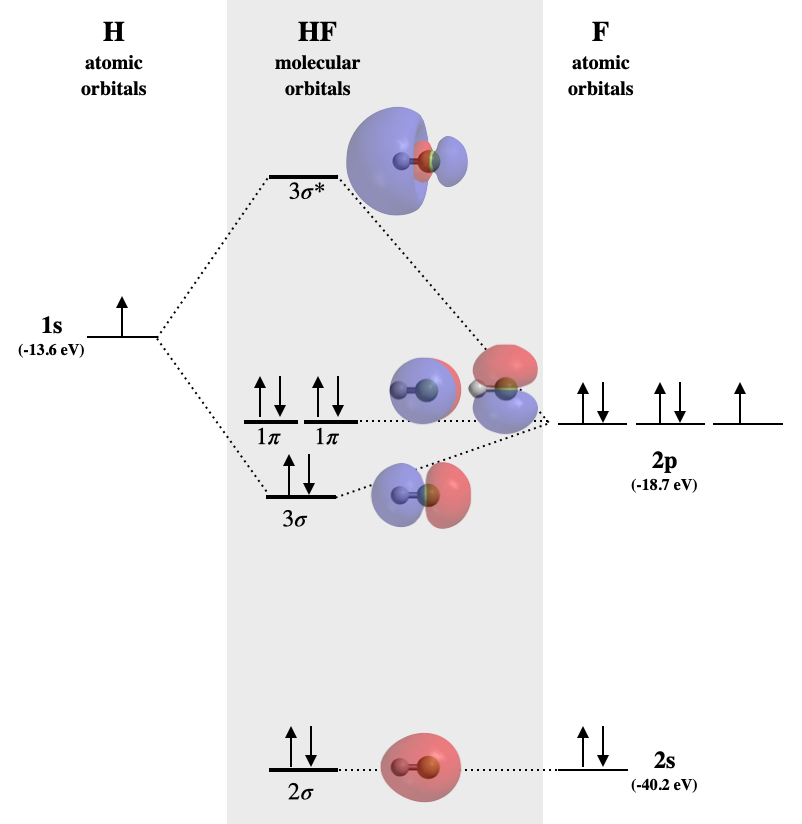
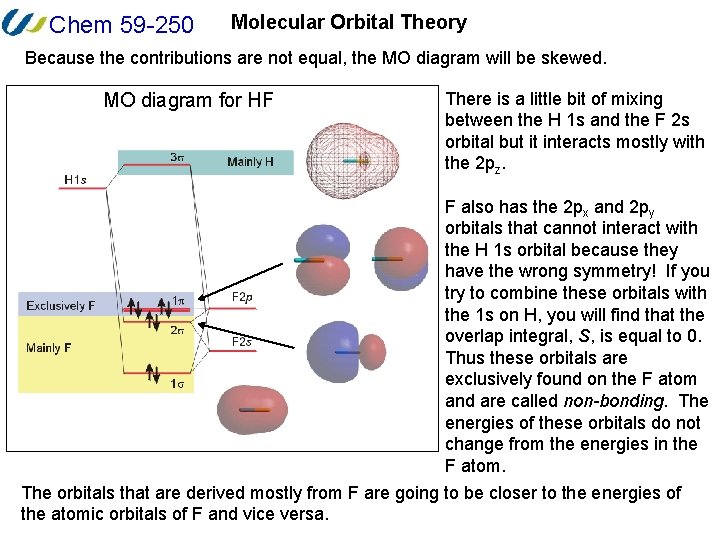
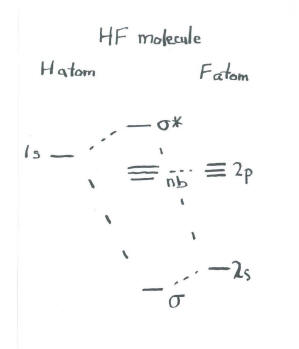
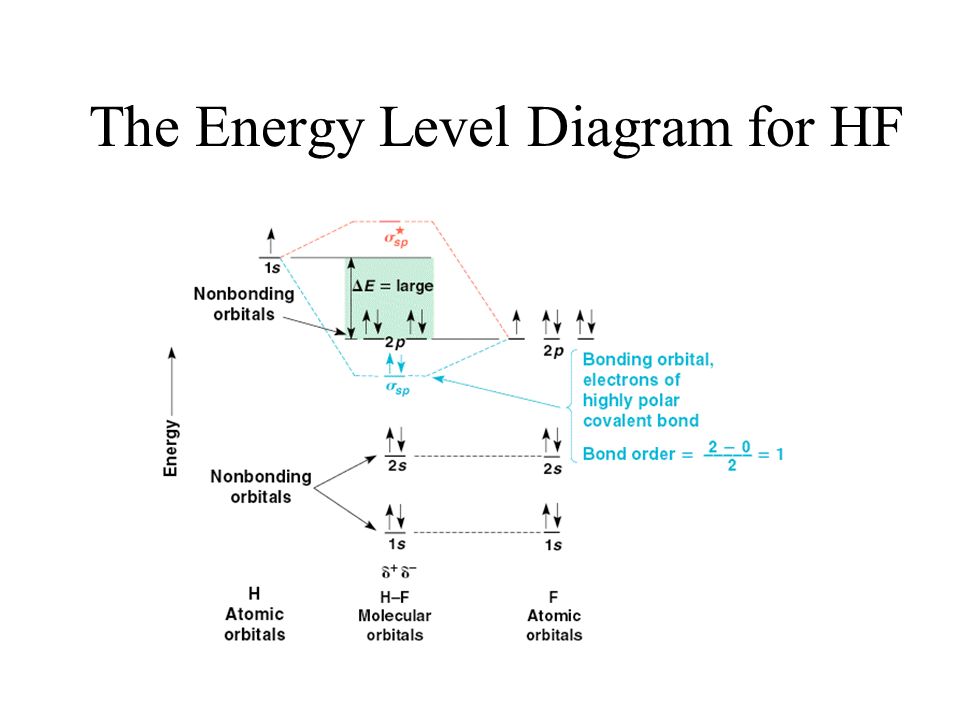
Solved Construct the molecular orbital diagram for HF. Use ... Construct the molecular orbital diagram for HF. Use only the valence electrons for your diagram. The 2s orbital of F atom has an energy more than 26 eV lower than that of the H 1s, so there is very little interaction between them. The F 2p orbital (-18.65 eV) and the H 1s (-13.61 eV), on the other hand, have similar energies, allowing them to

Molecular orbital diagram hf
MolecularOrbitals - Maple Help - Waterloo Maple In the LCAO approach, molecular orbitals are constructed by selecting sums and differences of atomic orbitals. For example, for hydrogen fluoride, we start with the H1s, F1s, F2s, F2px, F2py, and Fp2z orbitals. A linear combination of H1s and F2pz orbitals creates a bonding σ orbital and antibonding σ∗ orbital. Hf Molecular Orbital Diagram - Summarized by Plex.page ... This is also in the Lewis structure of HF molecule. + Charge indicates that the BMO / electron pair is shifted away from H and - indicates partial negative that is developed on F atom as result of the electron pair shifting towards it. We should continue more MO diagrams in upcoming posts. Be perpetua l student of and keep learning. Draw a molecular orbital diagram of N2 or O2 with magnetic ... Now, let us draw the molecular orbital diagram of ${N_2}$ . Now, first let us understand what magnetic behavior and bond order means. - Magnetic behavior: As we know the electron has an electron magnetic dipole moment, which is generally generated by the electron's spin property, which induces an electric charge into motion. As we can see the ...
Molecular orbital diagram hf. Constructing the HF molecular orbital energy level diagram ... In this screencast, Andrew Burrows walks you through how to construct the MO energy level diagram of HF. ... Molecular orbital diagrams for HBr and HF. | Download ... Molecular orbital diagrams for HBr and HF. Source publication +11 Total energy partitioning within a one-electron formalism: A Hamilton population study of surface-CO interaction in the c (2×2)-CO/... MO for HF - Chemistry LibreTexts A simple approach to molecular orbital (MO) theory for heterogeneous diatomic molecules is to show the energy level diagram. The MO energy levels can be worked out following these steps: Recall that the energy E n for the quantum number n is for an element with atomic Z is approximately (1) E n = 13.6 Z E f f 2 n 2 e V Molecular Orbital Diagram Of Lih basic idea behind molecular orbital theory - there are many variations on the bystep and deal with H2 and then Li2 and then LiH we will instead begin by. Molecular orbital energy level diagram for homonuclear diatomic molecules .. Density diagrams of the molecular orbitals for the LiH, CH, and HF molecules are .Molecular orbital.
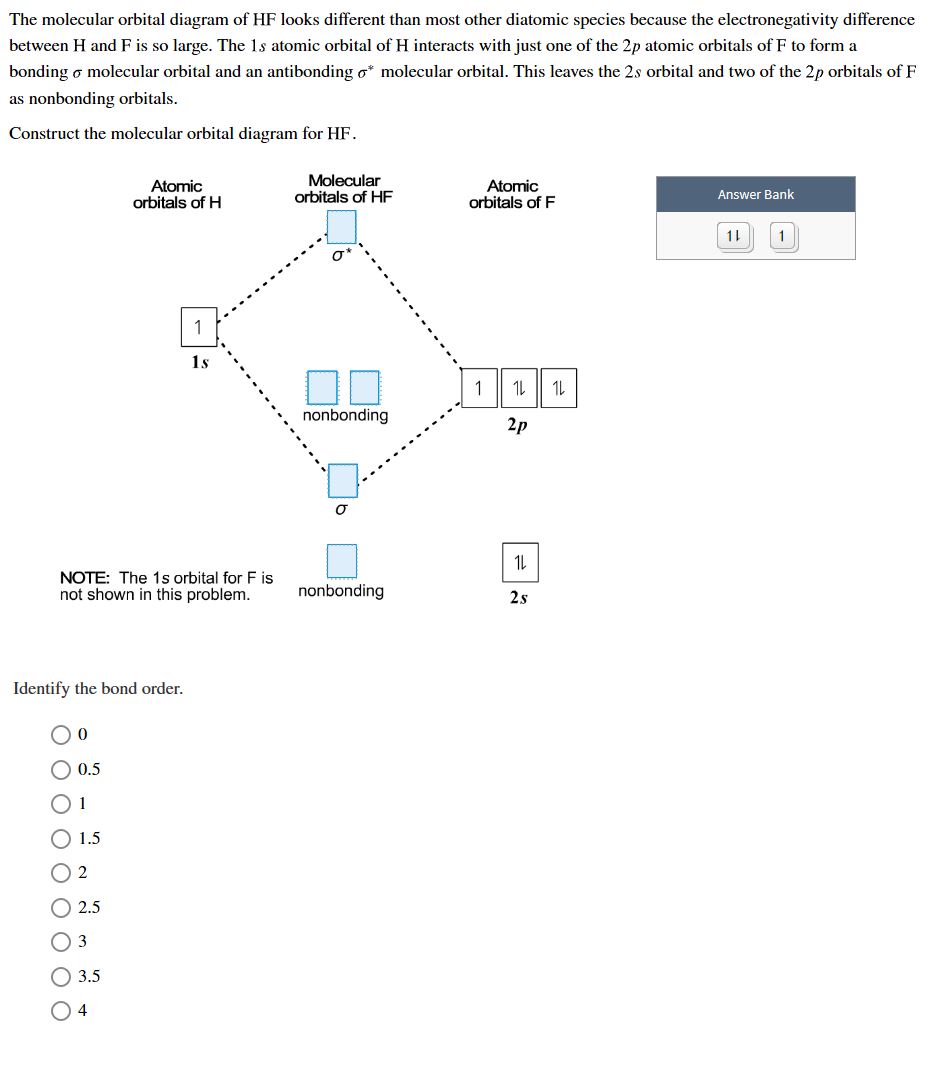
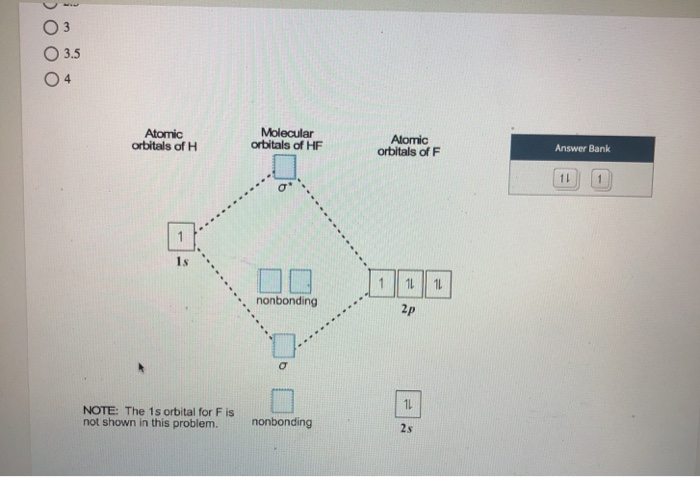
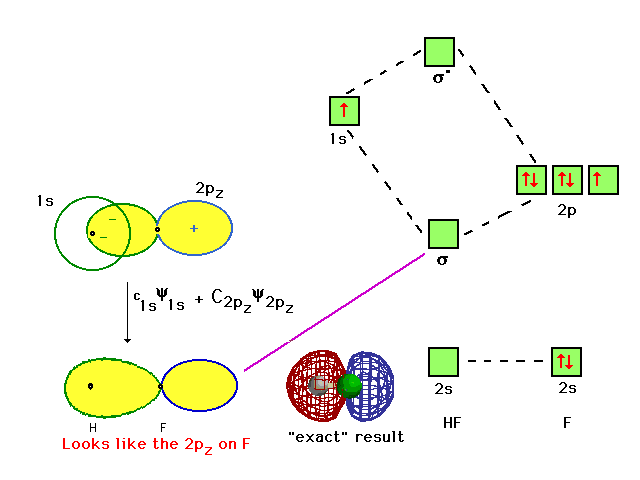
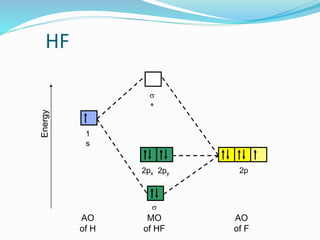
Molecular orbital diagram of HF and OH- - YouTube #MOT #BMO #ABMO #HF #CO #NO #CN #OHHello everyoneThis is shivam here To follow me on instagram search - Sshivam898To join telegram group click on the given l... Answered: The molecular orbital diagram of HF… | bartleby The molecular orbital diagram of HF looks different than most other diatomic species because the electronegativity difference between H and F is so large. The 1s atomic orbital of H interacts with just one of the 2p atomic orbitals of F to form a bonding o molecular orbital and an antibonding o molecular orbital. Molecular orbital diagram - Wikipedia Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels. Hbr Mo Diagram - Diagram World Molecular orbitals of HF. Draw the MO diagram for HBr including energy levels each orbitals shape each orbitals character meaning what atomic orbitals contribute to each MO. H1 is bonded in a linear geometry to two equivalent Br1- atoms. And how much symmetry labels eg. And how much symmetry labels eg.
Answered: "he molecular orbital diagram of HF… | bartleby The 1s atomic orbital of H interacts with just one of the 2p atomic orbitals of F to form a bonding o molecular orbital and an antibonding o* molecular orbital. This leaves the 2s orbital and two of the 2p orbitals of F as nonbonding orbitals. Construct the molecular orbital diagram for HF. Identify the bond order. 0.5 1 O 1.5 O 2 2.5 3 3.5 4 NCERT Solutions for Class 11 Chemistry Chapter 4 ... Free NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure solved by expert teachers from latest edition books and as per NCERT (CBSE) guidelines.Class 11 Chemistry Chemical Bonding and Molecular Structure NCERT Solutions and Extra Questions with Solutions to help you to revise complete Syllabus and Score More marks. Molecular Orbitals of Hydrogen Fluoride (HF) - Chemistry ... 1 Bearing in mind the energy and symmetry conditions, in the case of H F one can construct MOs using the 1 s AO on H and the 2 s and 2 p AOs on F. In general, the contribution to MOs is determined by the coefficients in the linear combination. Here, one observes that the 1 s electrons are almost completely localized on the F atom. Qualitative molecular orbital diagram of HF −. The 2σ ... Qualitative molecular orbital diagram of HF −. The 2σ orbital, coming from the F 2 s orbital, is nonbonding. The 3σ orbital is a combination of the F 2p z and H 1 s orbitals and is bonding ...
PDF Calculating the Energies of Molecular Orbitals much of each atomic coefficient is required to make each of the molecular orbitals. The coefficients for the highest energy MO wavefunction is solved below. Repeat these calculations for determining the coefficients of this molecular orbital, but then go on to calculate the coefficients for the other two MO's in our HF molecule.
Solved The molecular orbital diagram of HF looks different ... The molecular orbital diagram of HF looks different than most other diatomic species because the electronegativity difference between H and F is so large. The Is atomic orbital of H interacts with just one of the 2p atomic orbitals of F to form a bonding sigma molecular orbital and an antibonding sigma* molecular orbital.
CHEM129 Practice Problems Flashcards - Quizlet Start studying CHEM129 Practice Problems. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
How To Draw Molecular Orbital Diagram Of Co - Drawing ... A) draw a molecular orbital (mo) diagram for co and show the filling of electrons. Let's take [co (nh3)6]3+ as an example. For the homonuclear diatomic #o_2#, we simply have two copies of this atomic orbital diagram far apart at first. Electronic configuration of co molecule is: Draw the orbital diagram for the ion co2+.
Molecular Orbitals - Molecular Orbitals for Heteronuclear ... The molecular orbitals which involve ps orbitals are characteristically strongly polarized in a direction away from the bond in the region of the nucleus on which the p orbital is centred. Compare, for example, the 3 s orbitals of CH and HF with the 3 s g molecular orbital of the homonuclear diatomic molecules.
Module Two Chem 101 Problems Flashcards | Quizlet Start studying Module Two Chem 101 Problems. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Which is the molecular orbital diagram for HF? - Quora Molecular orbital energy level diagram of CO molecule can be given as. CO molecule has 10 valence electrons,four from carbon atom (2s²2p²) and six from oxygen atom (2s²2p⁴).According to molecular orbital diagram, molecular orbital configuration is given as σ2s² σ*2s² πx² πy² σz² π*x⁰ πy⁰ σ*z⁰ Thus , bond order = 1/2 (8-2)=3 83K views View upvotes
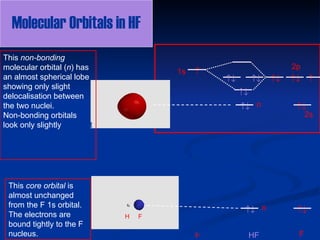
Molecular orbitals in Hydrogen Fluoride - ChemTube3D Home / Structure and Bonding / Atomic Orbitals / Molecular orbitals in Hydrogen Fluoride. Molecular orbitals in Hydrogen Fluoride. CONTROLS . Click on the HF molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules.
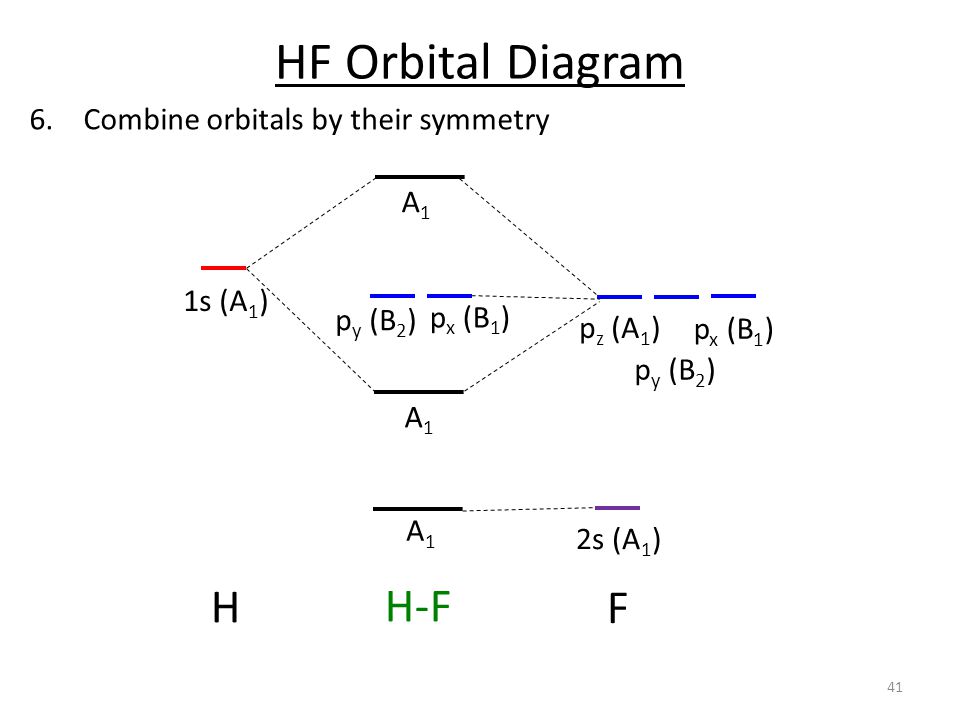
PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
Molecular orbital diagram of HF2-? | EduRev Chemistry Question This discussion on Molecular orbital diagram of HF2-? is done on EduRev Study Group by Chemistry Students. The Questions and Answers of Molecular orbital diagram of HF2-? are solved by group of students and teacher of Chemistry, which is also the largest student community of Chemistry.
Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagrams are diagrams of MO energy levels, shown as short horizontal lines in the center. Atomic orbitals (AO) energy levels are shown for comparison. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels. Levels with the same energy are commonly shown side by side.
HF - uwosh.edu HF HOMO Orbital. The HOMO orbital is the highest energy molecular orbital occupied by electrons. In HF, the HOMO orbitals are the double degenerate pi 2px and 2py and pi orbitals. To get a 3-D model you can manipulate, click here. Download time may be significant the first time the applet is loaded.
CS2 Molecular Geometry - Science Education and Tutorials Carbon disulfide(CS2) has the composition of one carbon and two sulfur atoms. What is the molecular geometry of carbon disulfide?. Drawing and predicting the CS2 molecular geometry is very easy by following the given method. Here in this post, we described step by step to construct CS2 molecular geometry. Sulfur and carbon come from the 16th and 14th family groups in the …
Molecular Orbital Diagram Of Lih - schematron.org we shall consider the molecular orbitals in lih, ch and hf to illustrate how molecular orbital theory describes the bonding in heteronuclear molecules, and to.the molecular orbital energy level diagram of lih in conventional textbooks for quantum chemistry is incorrect from viewpoint of ab initio hartree-fock scf-mo calculation, because the …
CN- lewis structure, molecular orbital diagram, and, bond ... Procedure to draw the molecular orbital diagram of CN 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 2. Find if the molecule homo-nuclear diatomic molecular orbital or hetero-nuclear diatomic molecular orbital. Clearly, CN is hetero orbital. 3.
Draw a molecular orbital diagram of N2 or O2 with magnetic ... Now, let us draw the molecular orbital diagram of ${N_2}$ . Now, first let us understand what magnetic behavior and bond order means. - Magnetic behavior: As we know the electron has an electron magnetic dipole moment, which is generally generated by the electron's spin property, which induces an electric charge into motion. As we can see the ...
Hf Molecular Orbital Diagram - Summarized by Plex.page ... This is also in the Lewis structure of HF molecule. + Charge indicates that the BMO / electron pair is shifted away from H and - indicates partial negative that is developed on F atom as result of the electron pair shifting towards it. We should continue more MO diagrams in upcoming posts. Be perpetua l student of and keep learning.
MolecularOrbitals - Maple Help - Waterloo Maple In the LCAO approach, molecular orbitals are constructed by selecting sums and differences of atomic orbitals. For example, for hydrogen fluoride, we start with the H1s, F1s, F2s, F2px, F2py, and Fp2z orbitals. A linear combination of H1s and F2pz orbitals creates a bonding σ orbital and antibonding σ∗ orbital.















0 Response to "36 molecular orbital diagram hf"
Post a Comment