37 silicon electron dot diagram
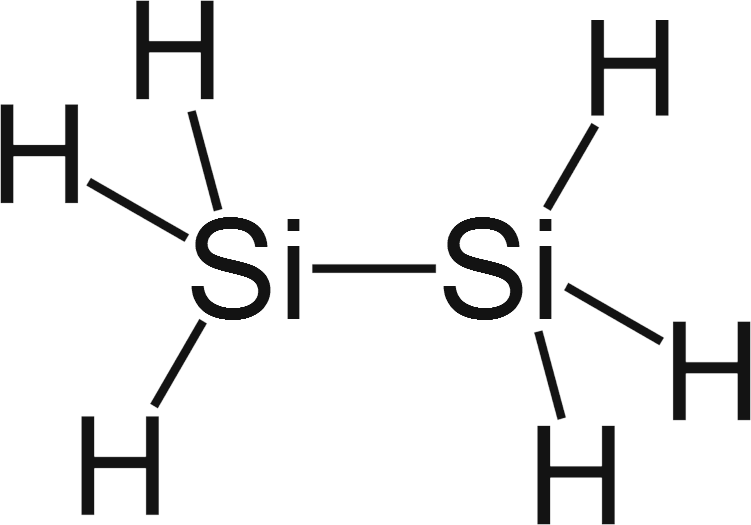
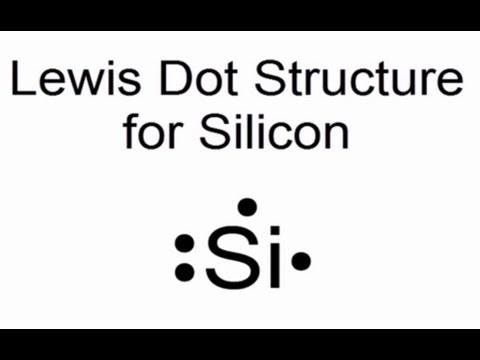
Silicon: Element Lewis Structure, Facts & Discovery ... Silicon is in group 14 and period 3 of the periodic table and has four valence electrons in its Lewis structure. The four valence electrons means that silicon can bond in a way similar to carbon ... What is the Lewis dot structure for silicon? How is it ... Answer (1 of 2): Apparently there are different schools of thought on this (How to Draw Electron Dot Diagrams). That said, it seems that the one followed (at least in the Western US) has you start with the right hand side and the S orbital (only the outer valence electrons!): Si: (easier to do t...
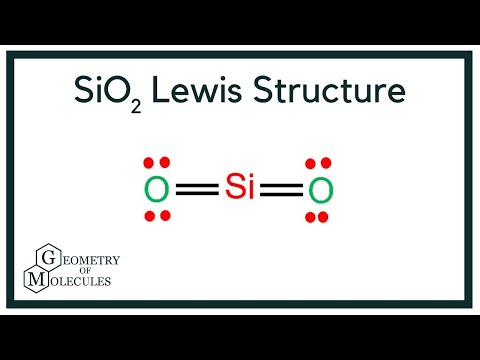
SiO2 Lewis Structure - Learnool The above structure is not a stable lewis structure because both silicon and oxygen atoms have charges. Therefore, reduce the charges (as below) by converting lone pairs to bonds. #4 Minimize Charges. Convert a lone pair of the oxygen atom to make a new Si — O bond with the silicon atom as follows:

Silicon electron dot diagram
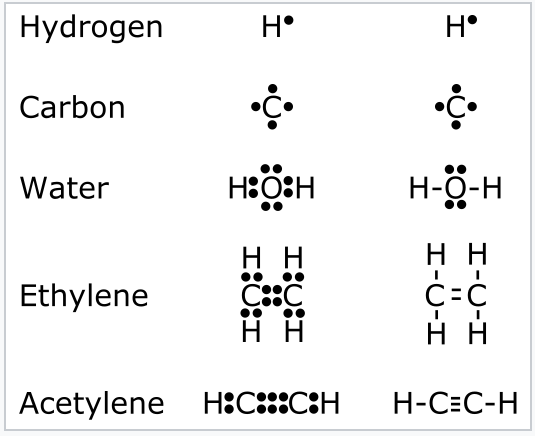
Lewis Structures ... 100+ Lewis Structures Check the Formal Charges to make sure you have the best Lewis Structure. Explain How Examples: SO 4 2-, N 2 O, XeO 3; Notable Exceptions to the Octet Rule. H only needs 2 valence electrons. Be and B don't need 8 valence electrons. S and P sometimes have more than 8 val. Electrons. Lewis Electron Dot Diagrams - GitHub Pages Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1. What is the electron dot diagram for neon? - AskingLot.com When you draw the Lewis structure for Silicon you'll put four "dots" or valance electrons around the element symbol (Si). Which is the electron dot structure of magnesium? Magnesium is element #12. To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s22s22p6).
Silicon electron dot diagram. SiO2 Lewis Structure, Molecular Geometry, Hybridization ... SiO2 has a net dipole moment of zero. It has a linear electron and molecular geometry with a bond angle of 180 degrees and a hybridization of Sp. The Silicon dioxide Lewis structure has a total of 16 valence electrons. In the Lewis dot structure of SiO2, the formal charge is zero. If you have any doubts, please feel free to ask in the comments ... How to make a Lewis structure out of SiC when both (Si and ... Answer (1 of 7): Silicon carbide (rarely: the mineral moissanite) is a refractory solid with a number of different allotropic covalent network structures. All of them have the atoms bound to four neighbors in a tetrahedral fashion with four covalent \sigma-bonds to the neighboring atom. One struc... What is the electron dot diagram for silicon? - Answers There are two types of diagrams one is the Lewis diagram the other is the Electron dot diagram. To make the electron dot diagram you put the electron symbol and put a dot on one of the sides for ... What is the electron dot structure for silicon? - Answers There are four valance electrons in Silicon, therefore there will be 4 dots in your electron dot diagram.
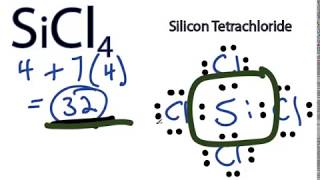
SiCl4 Lewis Structure - Learnool SiCl4 Lewis Structure. March 10, 2022 March 9, 2022 by Admin. SiCl 4 (silicon tetrachloride) has one silicon atom and four chlorine atoms. In the lewis structure of SiCl 4, there are four single bonds around the silicon atom, with four chlorine atoms attached to it, and on each chlorine atom, there are three lone pairs. Lewis Electron Dot Diagrams - Introductory Chemistry - 1st ... A. Lewis electron dot diagram. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... Lewis Dot Diagram For Tellurium - schematron.org Write the electron dot (Lewis) diagrams for the following. 9. carbon silicon Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Exercises Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. Silicon Bohr Model - How to draw Bohr diagram for Silicon ... Electron dot diagram of a Silicon atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Silicon, we got to know, it has only 4 valence electrons. So, just represent the 4 valence electrons around the Silicon atom as a dot.
Hee Sun drew an electron dot diagram of a silicon atom as ... Hee Sun drew an electron dot diagram of a silicon atom as shown. In addition to changing the symbol to C, how would this diagram. compare with an electron dot diagram of a carbon atom (C)? The dot to the left of the symbol would be removed in the diagram for carbon. The same number of dots would appear in the diagram for carbon. Silicon tetraiodide | SiI4 - PubChem Silicon tetraiodide | SiI4 or I4Si | CID 83498 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological ... 6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... SiS2 Lewis Structure, Molecular Geometry, Hybridization ... Lewis structure of Silicon disulfide (SiS2) Before studying the Lewis structure of Silicon disulfide, it is crucial to analyze the Lewis structures of participating atoms which are Silicon and Sulfur. The atomic number of Silicon is 14, and the electronic configuration is 1s2 2s2 2p6 3s2 3p2.
Silicon carbide | SiC - PubChem Silicon carbide | SiC or CSi | CID 9863 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities ...
SiCl4 Lewis structure, Molecular geometry, Bond angle ... SiCl4 lewis's structure is straightforward and very easy to draw. "Lewis diagram describes the chemical bonding of atoms within a molecule". Lewis structure of SiCl4 contains 12 lone pairs on surrounding atoms and zero on the central atom. There are 4 bonding pairs present in the lewis structure of Silicon tetrachloride.
What is the electron dot diagram for aluminum ... What is electron shell diagram? For that, we have electron shell diagrams. For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element.
SiO2 Lewis Structure| Step By Step Construction - What's ... SiO2 Lewis Structure (Step by Step Construction) In the SiO 2 lewis Structure, the overall ratio of silicon to the oxygen atom is 1:2.The Silicon oxygen bonds are strong and keep the atoms firmly in place. Following are the steps to construct the SiO 2 Lewis Structure.. Step-1: Count the valence electrons of atoms For the SiO 2 Lewis structure, we need to figure out the number of valence ...
Which model represents the electron dot diagram of silicon? L 1.4.2 Quiz: Scientific Practices Question 1 of 10 In a controlled experiment, which group experiences the test? O A. Measurements and observations O …. B. Experimental group O c. Control group O D. Instruments and equipment. sodium chloride has a boiling point of what degree celcius. 18) Identify compound (C) in the following synthetic ...
SiH4 Lewis Structure - How to Draw the Lewis Structure for ... A step-by-step explanation of how to draw the SiH4 Lewis Dot Structure (Silicon Tetrahydride).For the SiH4 structure use the periodic table to find the total...
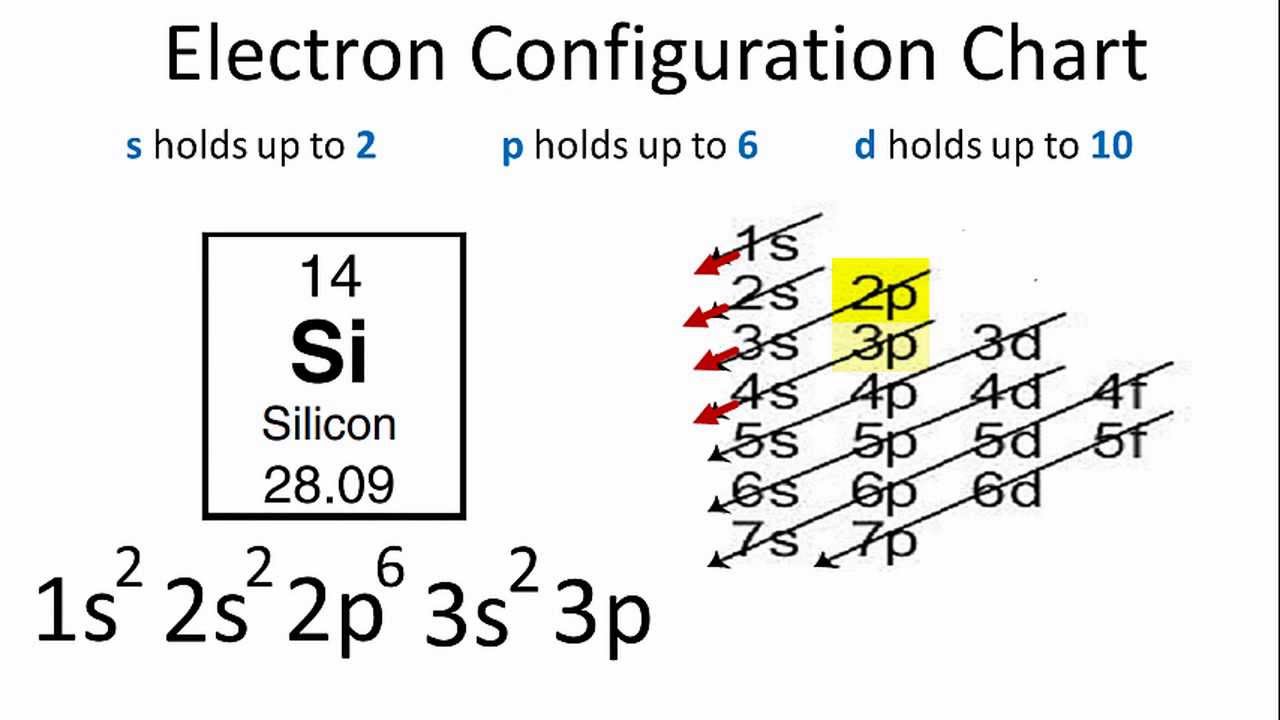
Electron Configuration for Silicon (Si) - UMD Therefore the Silicon electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 2. Video: Silicon Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to ...
Drawing Lewis diagrams (video) - Khan Academy And then the silicon is able to share in four bonds. Each of those bonds have two electrons, so the silicon is also feeling good about the octet rule. So I would feel very confident in this being the Lewis diagram, sometimes called the Lewis structure, for silicon tetrafluoride.
The Lewis structure for silicon disulfide is to be ... The Lewis structure for silicon disulfide is to be predicted. Concept introduction: The strategy for drawing Lewis structure is mention below. Calculate the number of valence electrons present in the molecule. Calculate the electron pairs by diving number of valence electrons by 2. Determine the bond pairs. Determine the lone pairs.
Lewis Dot Structure for Silicon Atom (Si) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for Si (Silicon). I show you where Silicon is on the periodic table and how to determine h...
What is the electron dot diagram for neon? - AskingLot.com When you draw the Lewis structure for Silicon you'll put four "dots" or valance electrons around the element symbol (Si). Which is the electron dot structure of magnesium? Magnesium is element #12. To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s22s22p6).
Lewis Electron Dot Diagrams - GitHub Pages Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1.
Lewis Structures ... 100+ Lewis Structures Check the Formal Charges to make sure you have the best Lewis Structure. Explain How Examples: SO 4 2-, N 2 O, XeO 3; Notable Exceptions to the Octet Rule. H only needs 2 valence electrons. Be and B don't need 8 valence electrons. S and P sometimes have more than 8 val. Electrons.









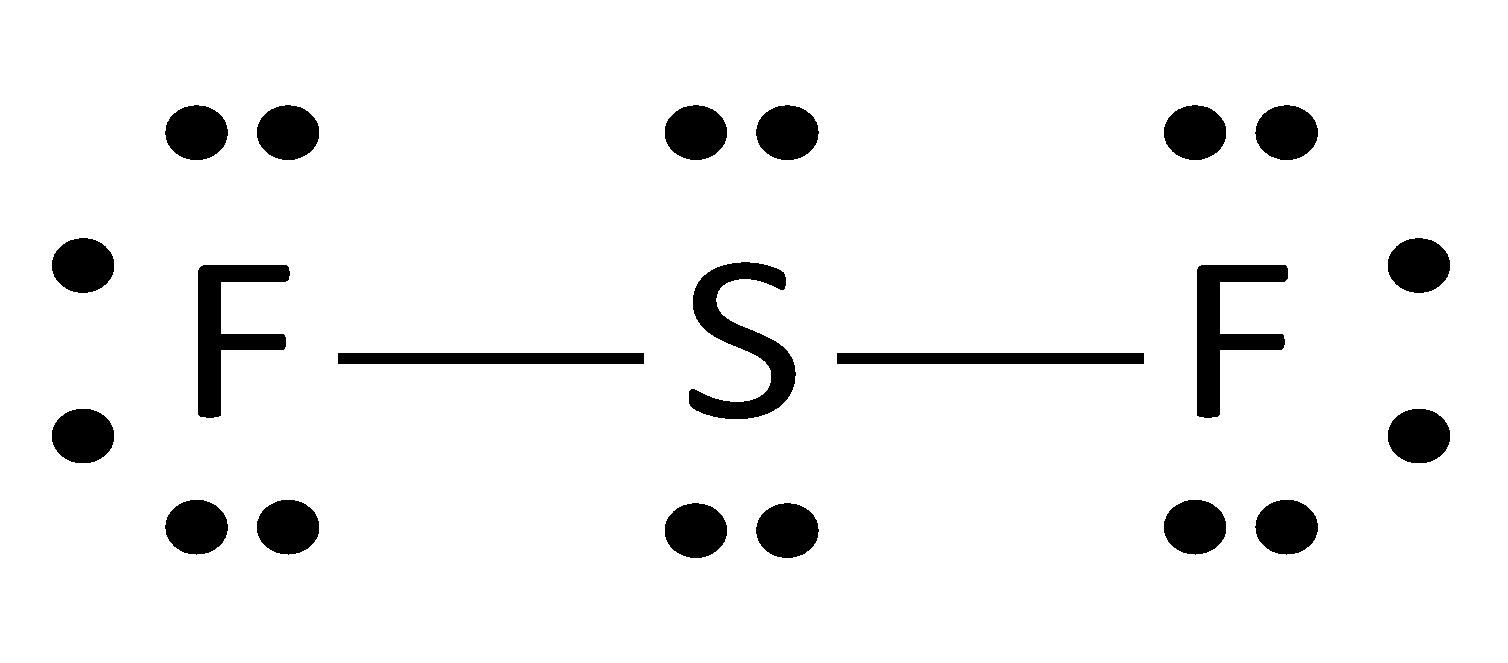





0 Response to "37 silicon electron dot diagram"
Post a Comment