40 atom energy level diagram
A number of energy-level diagrams are represented in Figs. 70-1 through 7c-15. ... Energy-level diagram of He 1-simplest atom with two valence electrons.14 pages 18:26This lesson focus on the arrangement of the electrons around the atom. It also looks at the work of Niels Bohr ...16 Sep 2014 · Uploaded by Mindset
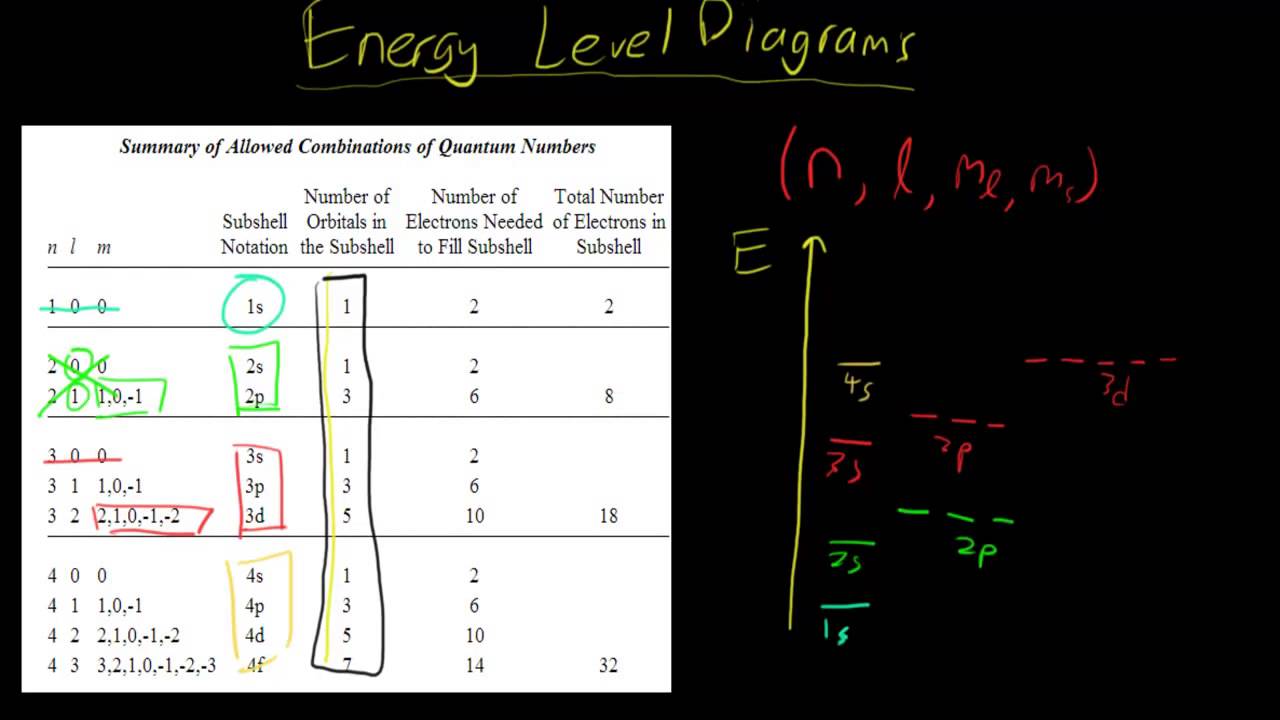
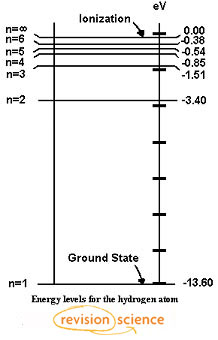
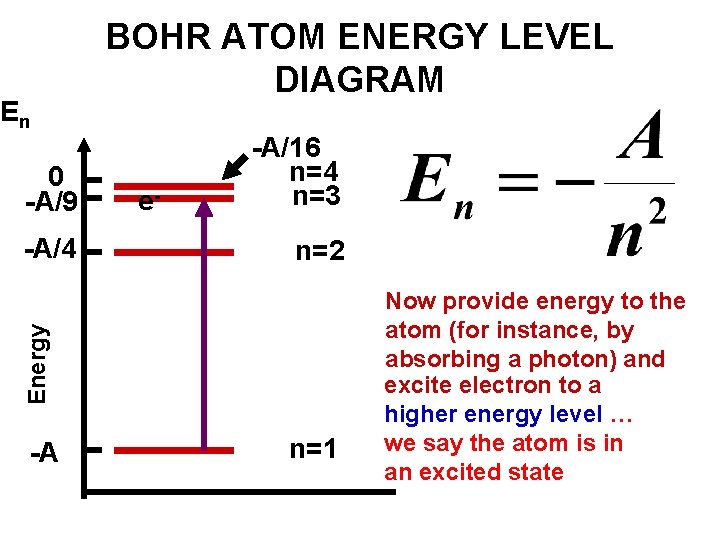
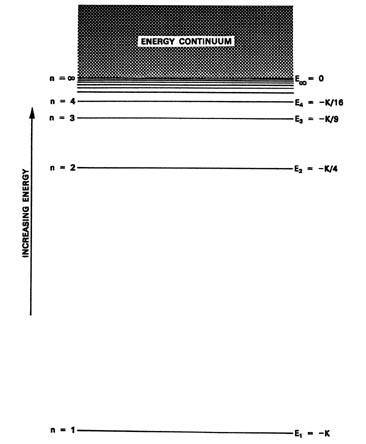
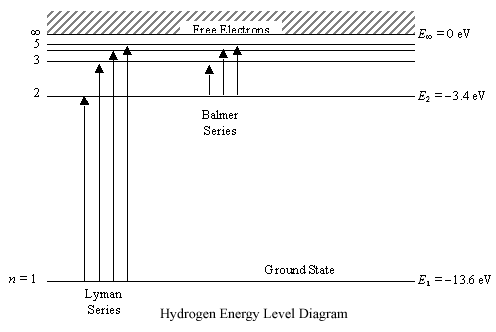
Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from ...
Atom energy level diagram
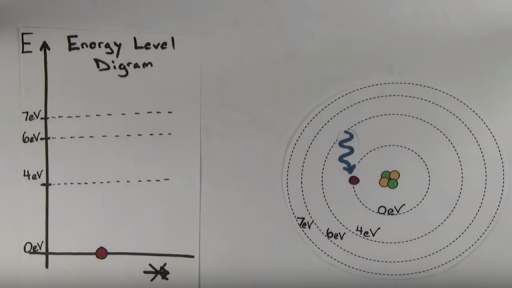
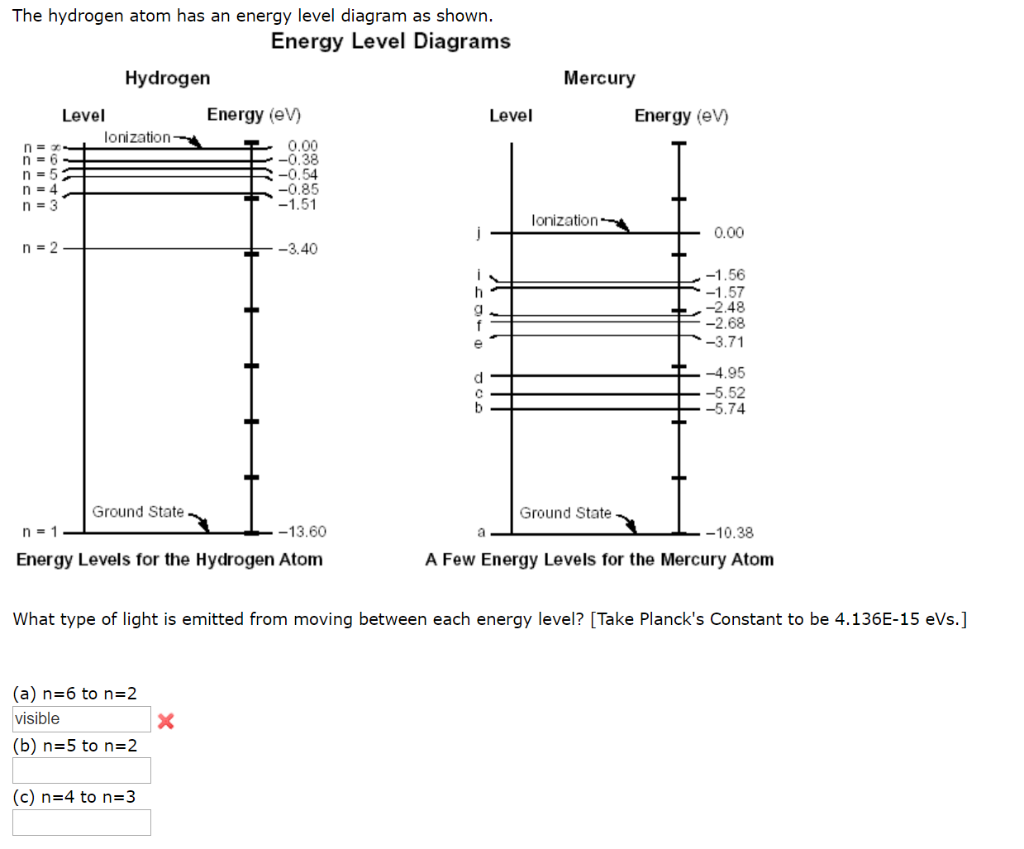
The diagram below shows the energy level diagram of a hydrogen atom. The associated spectrum to the diagram above is shown below. The transition labelled A in the top diagram gives the spectral line labelled B in the spectrum diagram. (a) (i) Show that the frequency of spectral line B is about 4.6 × 10 14 Hz. ΔE = 3.40-1.51 = 1.89 eV. The energy of an electron when it is far away from the influence of the nucleus is taken as zero. Principal quantum number of an electron existing in such a stationary state is taken as, n = ∞. Such kind of hydrogen atom is called an ionized hydrogen atom. A negative sign is placed in the above equation as, due to the transition of an ... The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Let's say our pretend atom has electron energy levels of zero eV, four eV, six eV, and seven eV. Note that moving left or right on an energy level diagram doesn't actually represent anything ...
Atom energy level diagram. Physics questions and answers. (Figure 1) is an energy-level diagram for a quantum system. Part A What wavelengths appear in the system's emission spectrum? Express your answers in nanometers separated by commas. VO ΑΣΦ ? 828.75,310.78,407.25 nm Figure 1 of 1 Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining ... What is energy level diagram? In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. The closest shell to the nucleus is called the “K shell” followed by the “L shell” then the “M shell” and so on away from the nucleus. The shells can be denoted by alphabets (K, L ... Three energy levels P, Q, R of a certain atom are such that E P < E Q < E R . If λ 1 , λ 2 a n d λ 3 are the wavelengths of radiation corresponding to transition R → Q; Q → P a n d R → P respectively. The correct relationship between λ 1 , λ 2 a n d λ 3 is: Energy level diagrams — There are various types of energy level diagrams for bonds between atoms in a molecule. Examples: Molecular orbital diagrams, ...
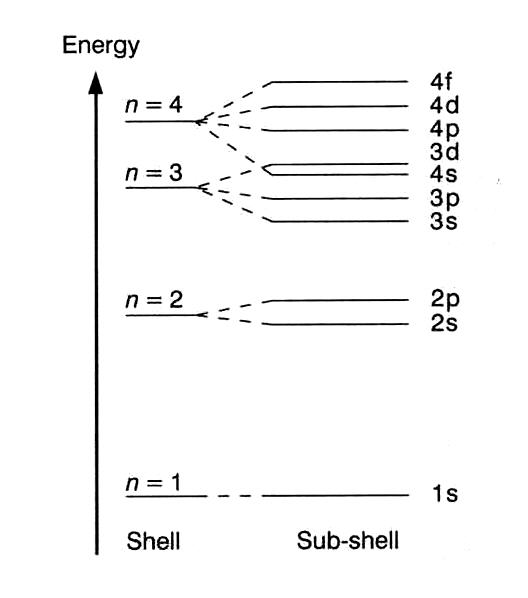
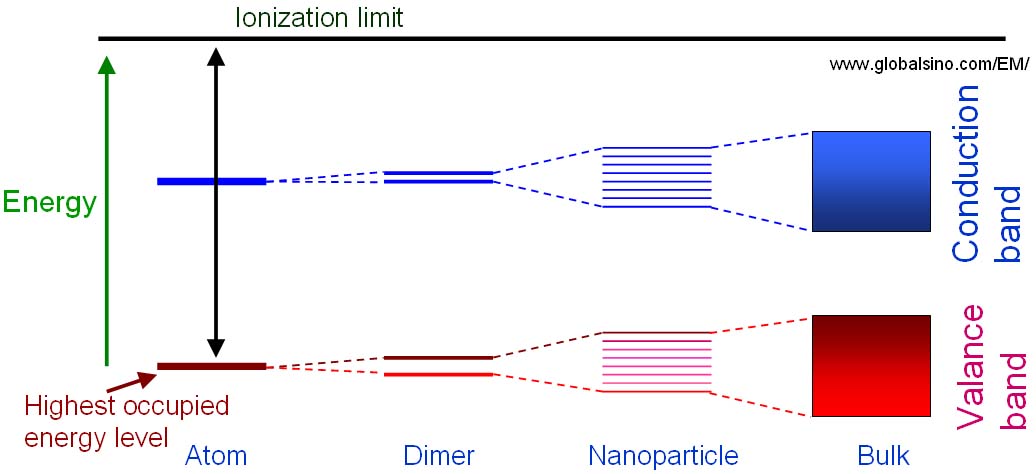
Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another. Suppose you want to draw the energy level diagram of oxygen. You look on the periodic table and find that oxygen is atomic number 8. This number means that ... Energy level diagrams for IONS Atoms with 1, 2, or 3 valence electrons lose them to form 1+, 2+ or 3+ ions respectively. naming metallic ions - the full name of the atom is followed by the word ion. Mg2+ is the magnesium ion Group 1 (1+) (lose 1e) Group 2 (2+) (lose 2e) Group 13 (3+) (lose 3e) Energy level diagrams for IONS Energy level diagrams can be useful for visualizing the complex level structure of multi-electron atoms. Forms of such diagrams are called Grotrian diagrams ...
PhysicsLAB: Energy-Level Diagrams. Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another. These energies differences correspond to the wavelengths of light in the discreet spectral lines emitted by an atom as it goes through de-excitation or by the ... 33. 0. The diagram shows some of the energy levels of an electron in a mercury atom. Level Q represents the lowest possible energy level. (a)Explain why a line spectrum results from an atom with such energy levels. (b) Calculate the energy change in joules when the electron moves from level P to level Q and determine the wavelength of the ... The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Let's say our pretend atom has electron energy levels of zero eV, four eV, six eV, and seven eV. Note that moving left or right on an energy level diagram doesn't actually represent anything ... The energy of an electron when it is far away from the influence of the nucleus is taken as zero. Principal quantum number of an electron existing in such a stationary state is taken as, n = ∞. Such kind of hydrogen atom is called an ionized hydrogen atom. A negative sign is placed in the above equation as, due to the transition of an ...
The diagram below shows the energy level diagram of a hydrogen atom. The associated spectrum to the diagram above is shown below. The transition labelled A in the top diagram gives the spectral line labelled B in the spectrum diagram. (a) (i) Show that the frequency of spectral line B is about 4.6 × 10 14 Hz. ΔE = 3.40-1.51 = 1.89 eV.

Energy State Hydrogen Energy States Kids Encyclopedia Children S Homework Help Kids Online Dictionary Britann Energy Energy Level Learning Worksheets

Draw A Neat Labelled Diagram Showing Energy Levels And Transition Between Them For The Hydrogen Atoms Physics Shaalaa Com
For The Following Pairs Of Electron Transition Which Produces The Emission With Longest Wavelength Give Rationale Behind Your Answer A N 3 N 1 Versus N 2 N 1 B 3p 2s Versus 2p 1s

The Figure Shows An Energy Level Diagram For The Hydrogen Atom Several Transitions Are Marked Youtube

Energy Quantisation And Electron Configuration Siyavula Textbooks Grade 10 Physical Science Openstax Cnx
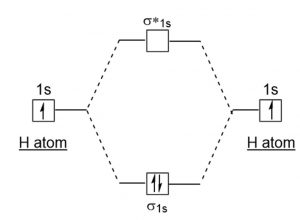
Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11





















0 Response to "40 atom energy level diagram"
Post a Comment