39 molecular orbital diagram for h2 and bond order
Hint: Generally the molecular orbital diagrams are used to understand the bonding of a diatomic molecule. You should know that molecular orbital diagrams are used to deduce magnetic properties of a molecule; they also help us to find out the bond order of the molecule. The orbital correlation diagram in predicts the same thing two electrons fill a single bonding molecular orbital. Molecular orbital diagram for h2. A the molecular orbital energy level diagram for the h2 molecule. B the shapes of the molecular orbitals are obtained by squaring the wave functions for mo1 and mo2.
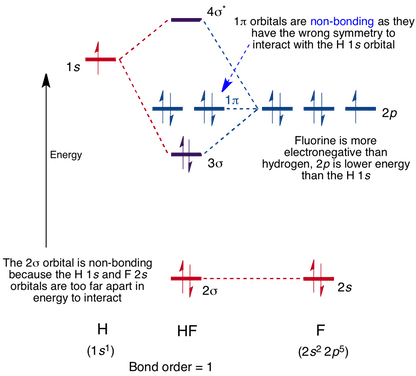
From the hydrogen, its valence 1s electron interacts with the 2p electrons of fluorine. This molecule is diamagnetic and has a bond order of one. Triatomic ...

Molecular orbital diagram for h2 and bond order
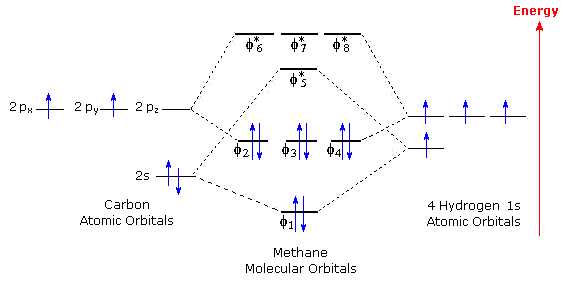
This video discusses how to draw the molecular orbital (MO) diagram for the H2+ ion. The bond order of H2+ is also calculated and the meaning of this number ... Description of the molecular orbitals of the h2 molecule with an introduction to molecular orbital diagrams. Each boron atom has one 2s and three 2p valence orbitals. In order to predict the bond order molecular orbital diagram for h2 is to be drawn. Molecular orbitals of h 2 the molecular orbital approach is one explanation for the ceh h bond. Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals.
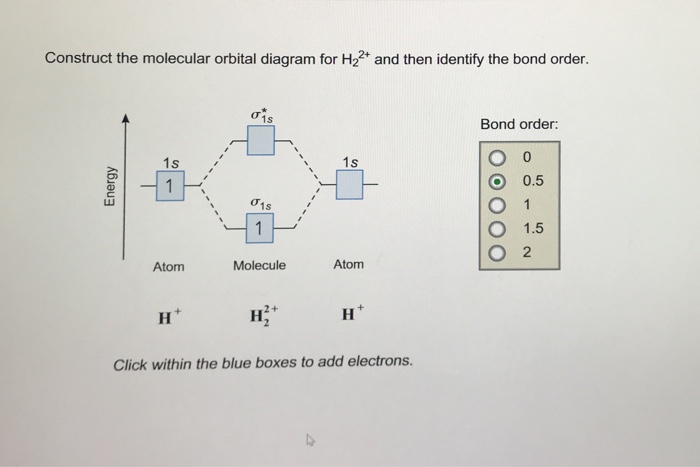
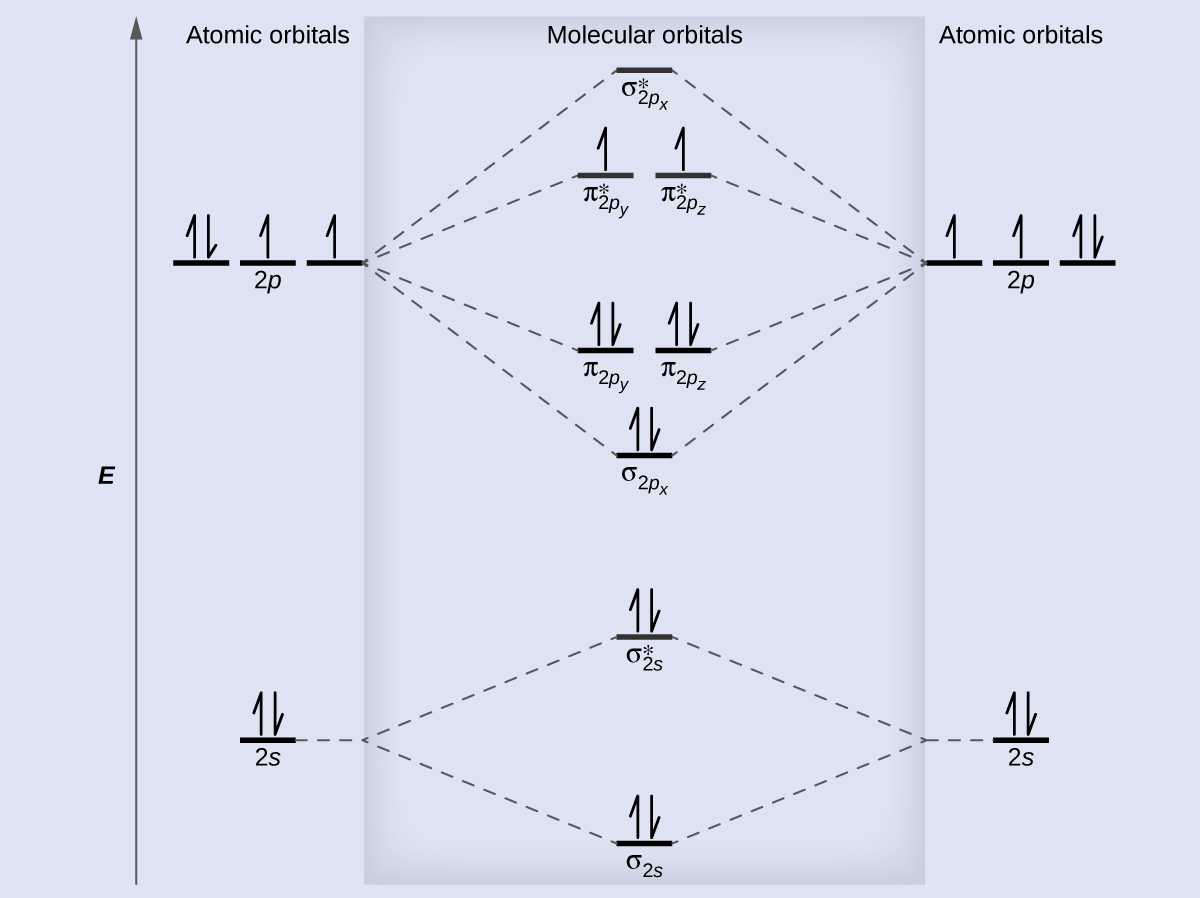
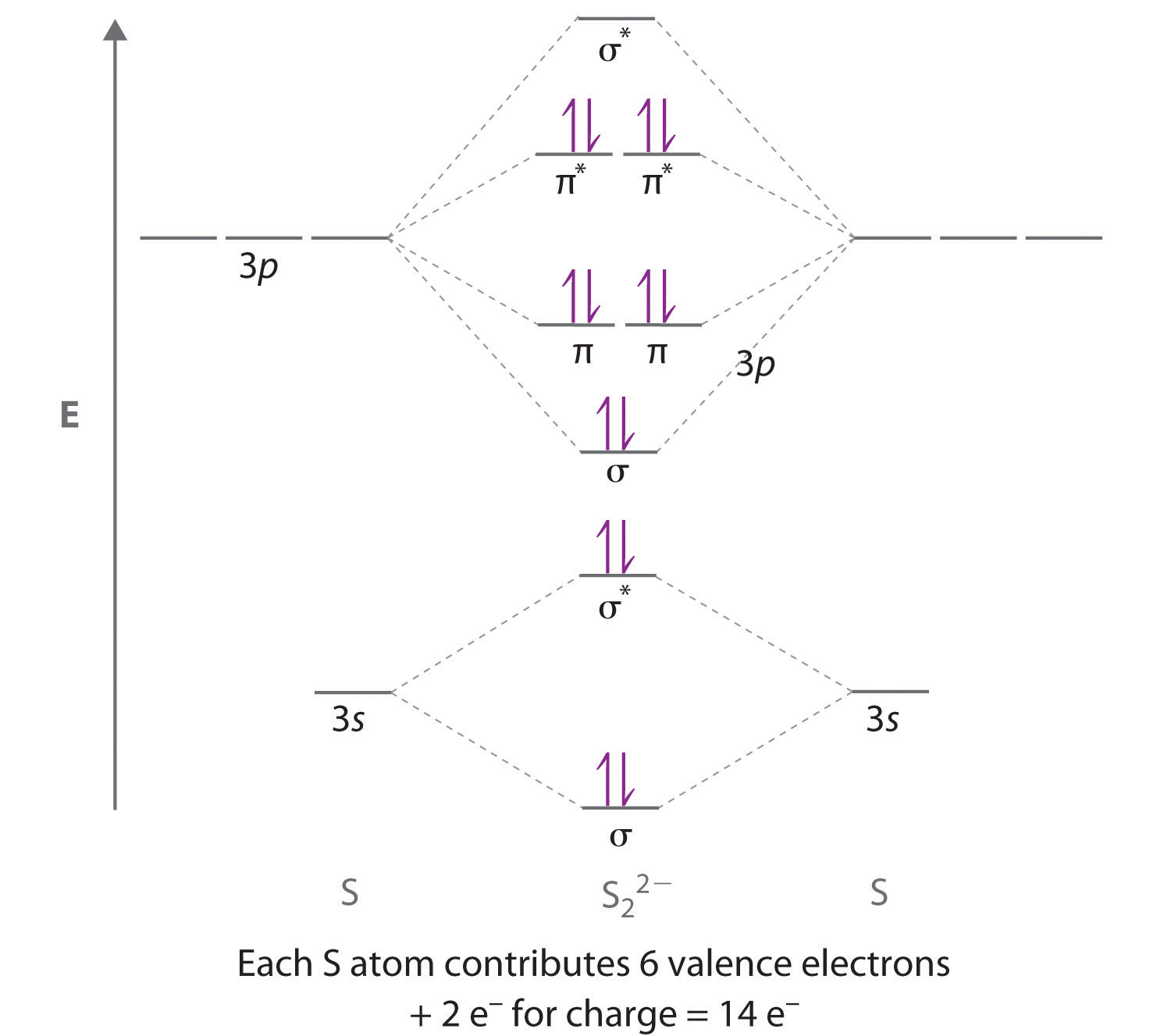
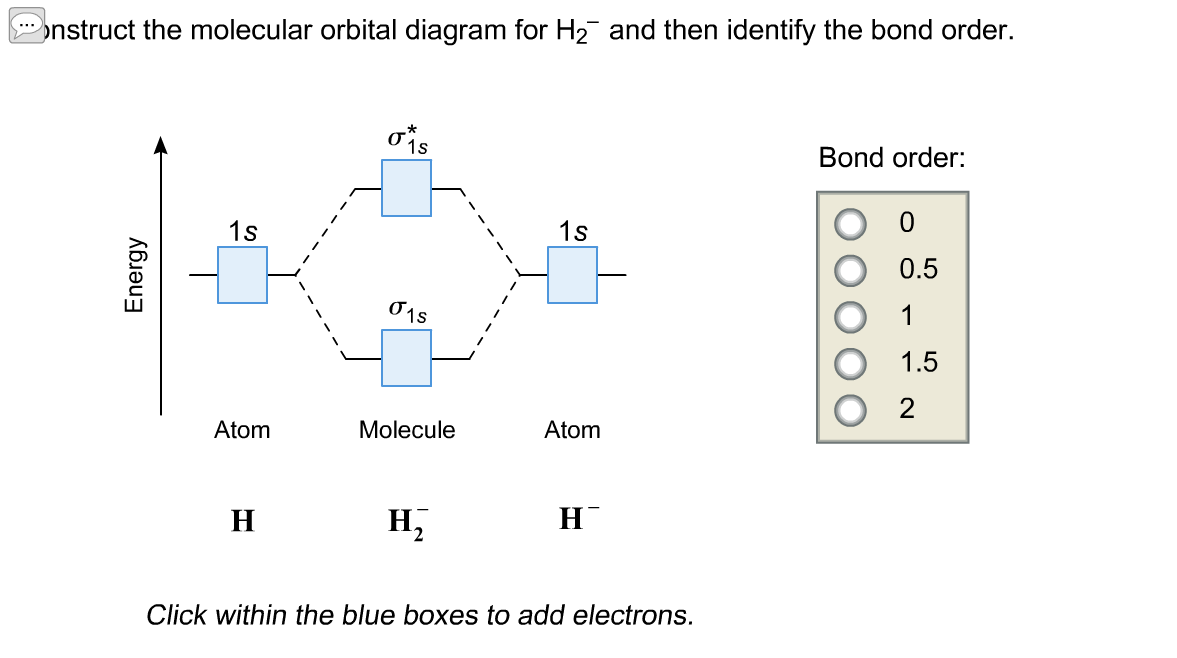
Molecular orbital diagram for h2 and bond order. Testin g qualitative MO theory prediction of Bond Order with experiment for homonuclear diatomics made from elements in the 1st row of the Periodic Table (using the "Molecular Orbital Aufbau" principle): BondOrder [# ' # ' ]/2≡−bondinge s antibondinge s [D.A. McQuarrie, Quantum Chemistry] Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... The bond order describes the stability of the bond. From the completed energy level diagram we can calculate the bond order defined as one half the net number of bonding electrons. 2 know that the higher the bond order the more stable the molecule. Molecular orbital diagram for h2 7. Bond order is the number of chemical bonds between a pair of ... This problem has been solved! A.Construct the molecular orbital diagram for H2^2+ and then identify the bond order. B.Construct the molecular orbital diagram for H2 and then identify the bond order. C.Construct the molecular orbital diagram for H2^- and then identify the bond order. D.Construct the molecular orbital diagram for H2^+ and then ...
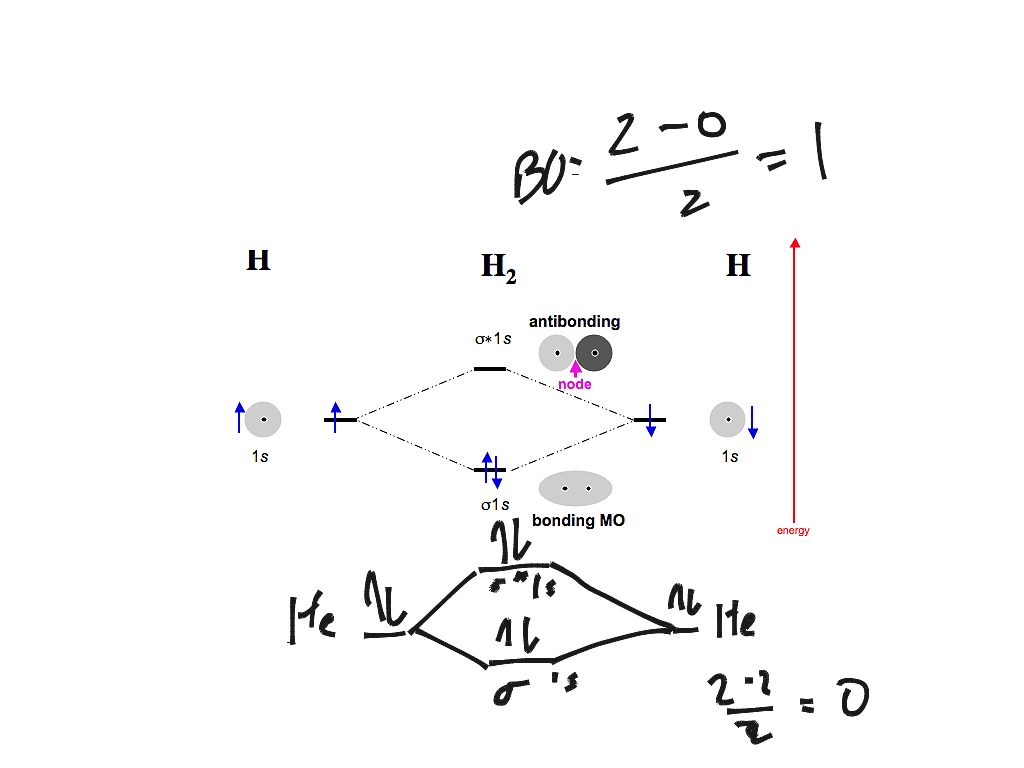
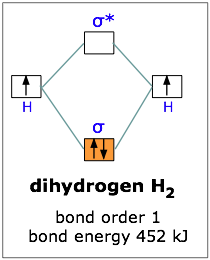
A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ... This question deals with the molecular orbital (MO) diagram of the simple diatomic hydrogen molecule. VSEPR theory describes this as two hydrogen atoms forming a single covalent bond. The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure 13. On either side of the central ladder are shown the energies of the 1 s orbitals of atoms A and B, and the central two-rung ladder shows the energies of the bonding and antibonding ... LUMO = lowest unoccupied molecular orbital HOMO = highest occupied molecular orbital Similar phase of electron density (no node) adds together constructively. energy of isolated atoms bond order (H2 molecule) = (2) - (0) 2 = 1 bond 1sb H H H H σ∗ = 1s H H a - 1sb = antibonding MO = LCAO = linear combination of atomic orbitals node = zero ...
the bond order is negative or zero. 3) Relative stability of molecule in terms of bond order. For diatomic molecules ,the stability is directly proportional to the bond order. A molecule with the bond order of 3 is more stable than a molecule with bond order of 2 and so on. 4) Nature of bond in terms of bond order : Bond order 1 ,2 and 3 mean ... Hydrogen atom's valence orbitals, before bonding, include every orbital, and all are the same energy for a specific n. At that point, there is only one electron. HAVING MORE THAN ONE ELECTRON SPLITS ENERGY LEVELS When introducing more electrons into the system, i.e. with another hydrogen wanting to bond, the repulsion splits the energy levels of the AOs of hydrogen. They start out where each n ... Construct the molecular orbital diagram for h2 and then identify the bond order. In fact they do. πε and jr k r mr lr defined explicitly in atkins. The orbital correlation diagram in predicts the same thing two electrons fill a single bonding molecular orbital. The procedure can be introduced by considering the h2 molecule. The bond order of a diatomic molecule is defined as one half the difference between the number of electrons in bonding orbitals nb and the number of electrons in antibonding orbitals na. O2 molecular orbital diagram oxygen has a similar setup to h 2 but now we consider 2s and 2p orbitals. Bonding order is 1 and it is diamagnetic.
Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration.
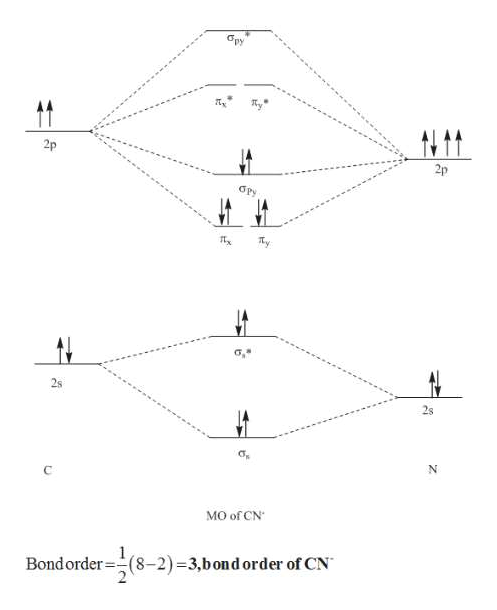
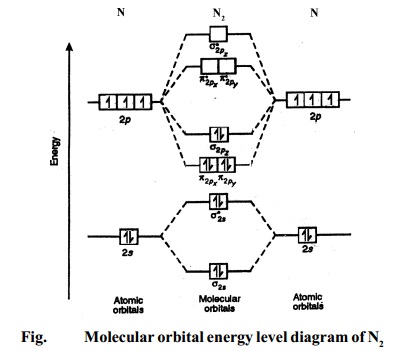
bond orderthe number of overlapping electron pairs between a pair of atoms. antibondingan atomic or molecular orbital whose energy increases as its constituent atoms converge, generating a repulsive force that hinders bonding. Bond order is the number of chemical bonds between a pair of atoms; in diatomic nitrogen (N≡N) for example, the bond ...
A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [(2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 .
Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me ...
Feb 3, 2021 — For H2, bond order = 1/2 (2-0) = 1, which means H2has only one bond. The antibonding orbital is empty. Thus, H2 is a stable molecule. Again, in ...
The bond order of a diatomic molecule is defined as one half the difference between the number of electrons in bonding orbitals nb and the number of electrons in antibonding orbitals na. A double covalent bond a bond order of two. Construct the molecular orbital diagram for h2 and then identify the bond order. Mo diagram of dihydrogen.
In molecular orbital theory bond order is defined as half of the difference between the number of bonding and antibonding electrons. The bond order of diatomic nitrogen is three and it is a diamagnetic molecule. Construct the molecular orbital diagram for h2 and then identify the bond order. According to mot number of atomic orbitals combined ...
Now we can add electrons to the diagram. Each hydrogen atom has 1 electron. These 2 electrons go to fill the lowest energy molecular orbital, the sigma bonding orbital. The electrons are more stable, lower energy, in the molecular orbital than they were in the separated atomic orbitals. In other words, when the H-H bond forms, each atom loses ...
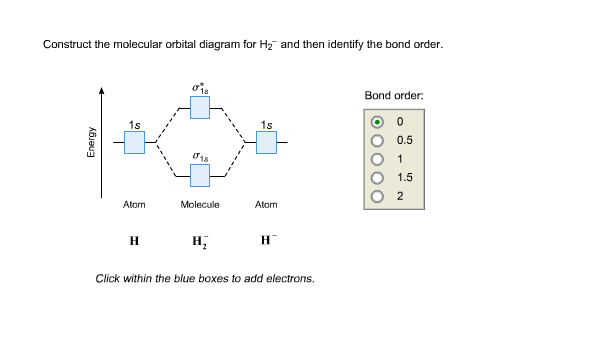
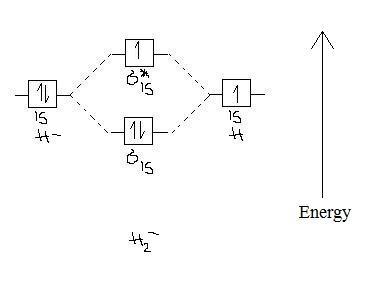
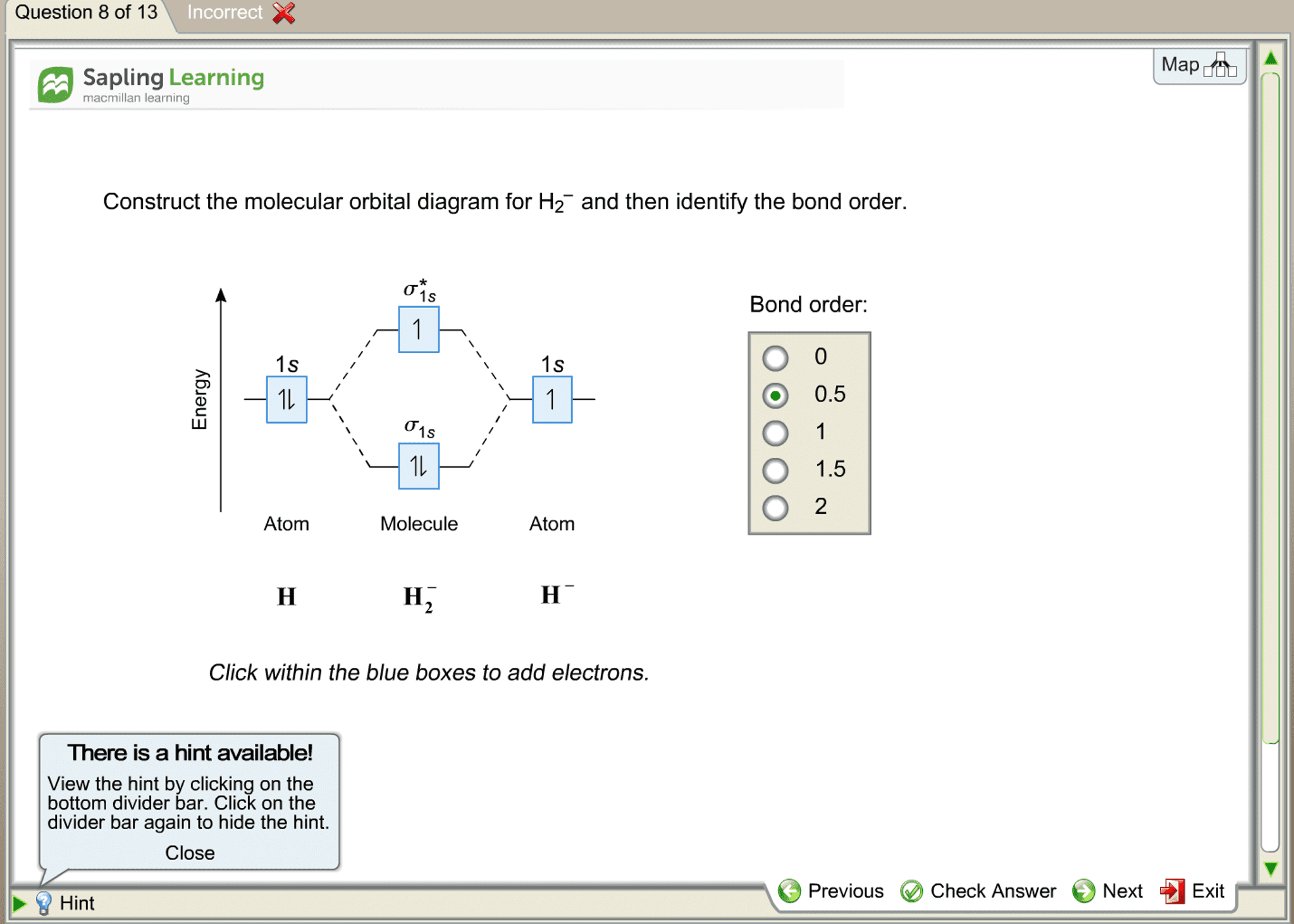
Science. Chemistry. Chemistry questions and answers. Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes to add electrons. Question: Construct the molecular orbital diagram for H2- and then identify the bond order.
Bonding mos antibonding mos and bond order. Molecular orbital diagram of h2. The electrons occupying the s h h orbital represent the bonding pair of electrons from the lewis structure of h2 and is aptly named a bonding molecular orbital. The procedure can be introduced by considering the h2 molecule. Molecular orbitals of h2 and he2.
Explain About The Molecular Orbital Diagram Of Hydrogen Molecule Sarthaks Econnect Largest Online Education Community
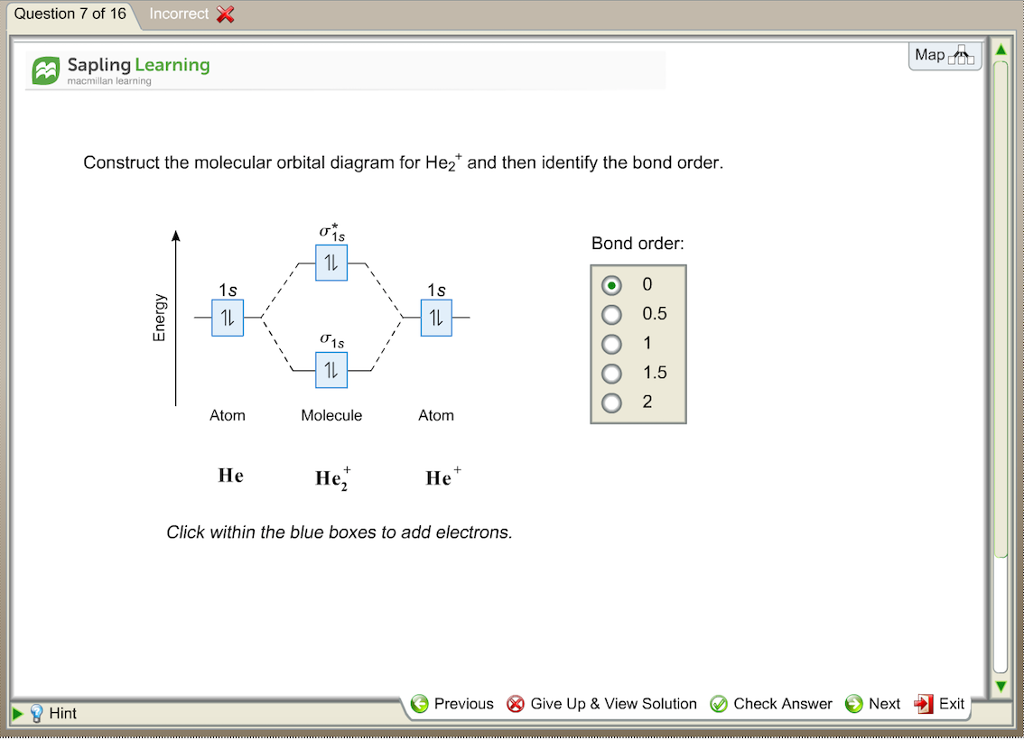
1. Problem: Draw MO energy diagrams for the molecular ions H2+ and H Since both molecular ions have a bond order of 1/2, they are approximately equally.Solution: Construct the molecular orbital diagram for He2 + and then identify the bond order. Problem Construct the molecular orbital diagram for He 2 + and then identify the bond order.
Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here. Chemical bonding - Molecular orbitals of H2 and He2: The procedure can ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.The Hydrogen Molecule Ion H2+Molecular Orbital Diagrams of Diatomic Molecules - Chem
Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals.
Description of the molecular orbitals of the h2 molecule with an introduction to molecular orbital diagrams. Each boron atom has one 2s and three 2p valence orbitals. In order to predict the bond order molecular orbital diagram for h2 is to be drawn. Molecular orbitals of h 2 the molecular orbital approach is one explanation for the ceh h bond.
This video discusses how to draw the molecular orbital (MO) diagram for the H2+ ion. The bond order of H2+ is also calculated and the meaning of this number ...

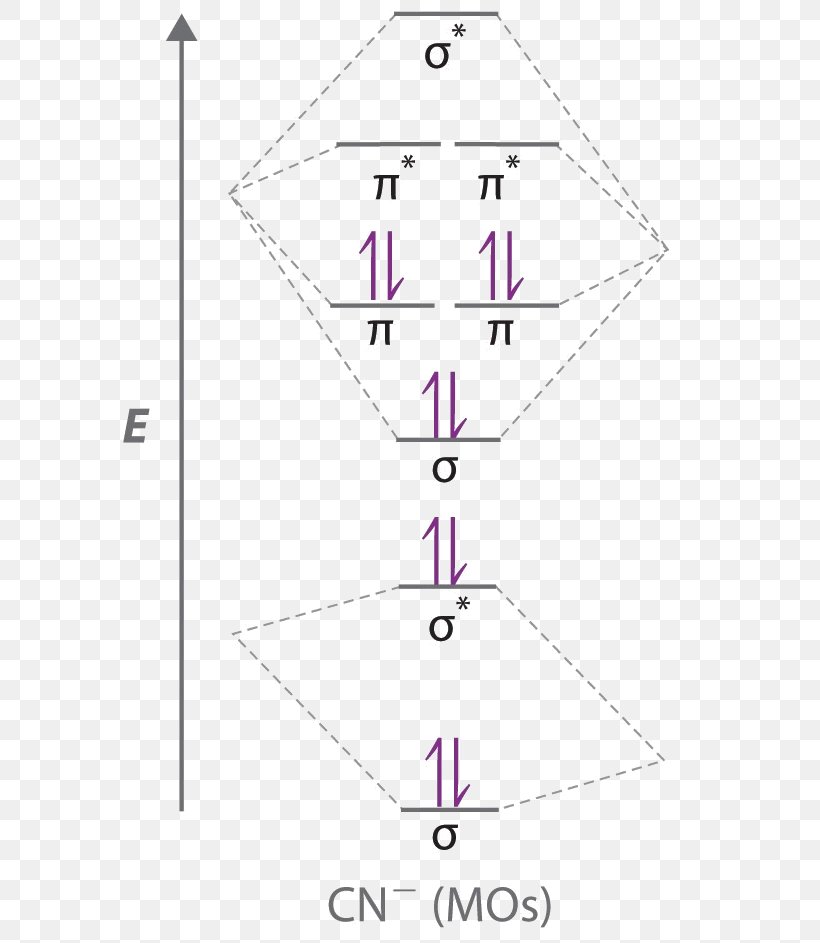
Consider The Molecular Orbital Diagram For The Ion O 2 2 Predict The Bond Order A 3 0 B 2 5 C 1 0 D 2 0 E 1 5 Consider The Following Statements Will The Ion Be Paramagnetic Or Study Com

Use Molecular Orbital Theory To Determine Whether He2 2 Or He2 Is More Stable Draw The Molecular Orbital Diagram For Each And Explain Study Com
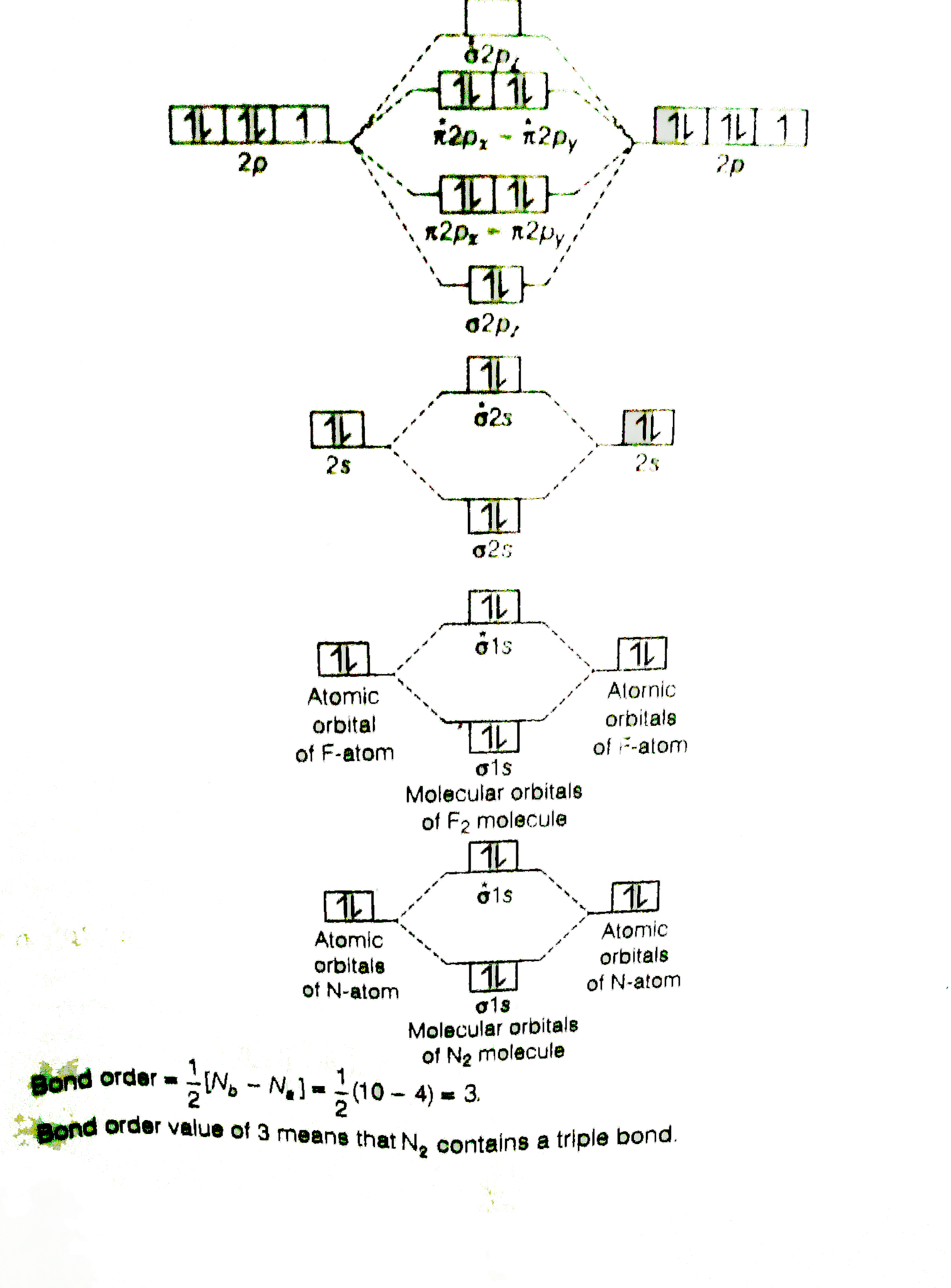
Use The Molecular Orbital Energy Level Diagram To Show That N 2 Would Be Expected To Have A Triple Bond F 2 A Single Bond And Ne 2 No Bond

Construct The Molecular Orbital Diagram For He2 And Then Identify The Bond Order Bond Order Click Homeworklib

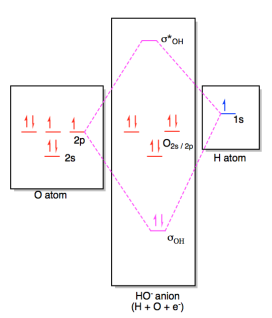






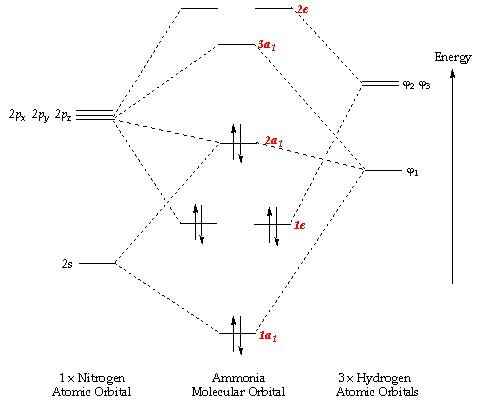




0 Response to "39 molecular orbital diagram for h2 and bond order"
Post a Comment