40 no+ mo diagram
NO Lewis Structure, Molecular Geometry ... - Techiescientist Take your NO molecule. As we can see. NO, or nitric oxide has two atoms: one N atom, and one O atom. Step 2. Nitrogen has five valence electrons in its outermost shell and Oxygen has six valence electrons. Therefore, total valence electrons in NO= 5+6 = 11 Step 3. Now, let us draw these electrons as dots surrounding the nitrogen and oxygen atoms. What is the electronic configuration of NO+? FUNDAMENTAL STEPS IN DERIVING MO DIAGRAMS Find the valence electron configuration of each atom in the molecule. Decide if the molecule is homonuclear of heteronuclear. Fill molecular orbitals using energy and bonding properties of the overlapping atomic orbitals. Use the diagram to predict properties of the molecule. What is bond order for no?
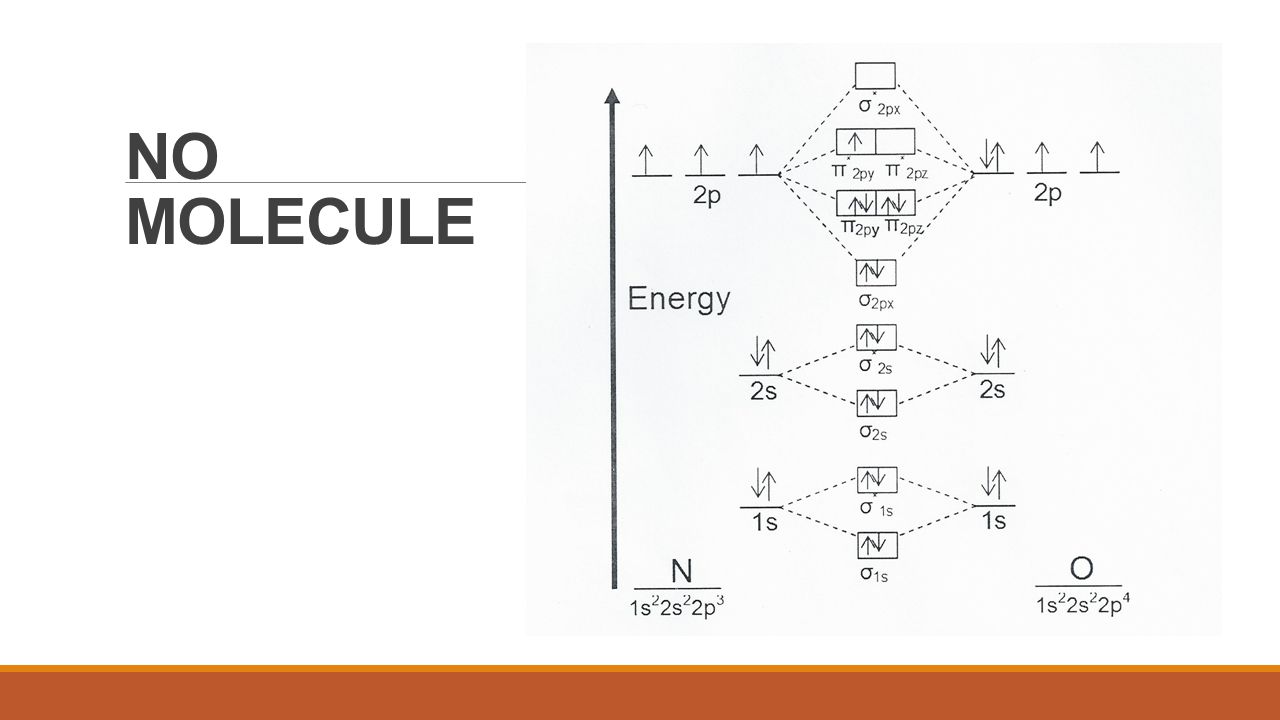
What is the molecular orbital diagram for NO? - Quora Your MO diagram for NO should look like this: O is more electronegative than N so its orbitals are slightly lower in energy and the bonding orbitals are slightly more concentrated on O. Note the odd electron is in a Pi*2p orbital. Now draw two more MO diagrams for NO+ and NO-

No+ mo diagram
NO, NO+ and NO-. Using the molecular orbital theory, how ... Your MO diagram for NO should look like this: O is more electronegative than N so its orbitals are slightly lower in energy and the bonding orbitals are slightly more concentrated on O. Note the odd electron is in a Pi*2p orbital. Now draw two more MO diagrams for NO+ and NO- Chemistry Community Nov 21, 2011 · Re: MO diagram for NO+. Postby Chem_Mod » Mon Nov 21, 2011 11:19 pm. Just draw the atomic orbitals of O lower. This will result in the antibonding molecular orbitals being closer to N. Top. 2 posts • Page 1 of 1. Return to “*Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)”. Jump to. energy - Chemistry Stack Exchange For this reason, I am reluctant to present a molecular orbital diagram. However, I will provide calculated MOs. While it may be tempting to just look at the pictures, the rationalisation behind it is more important. What will follow now are a few attempts at that rationalisation. In the nitric oxide series, $\ce{NO+}$ is surprisingly the simplest.
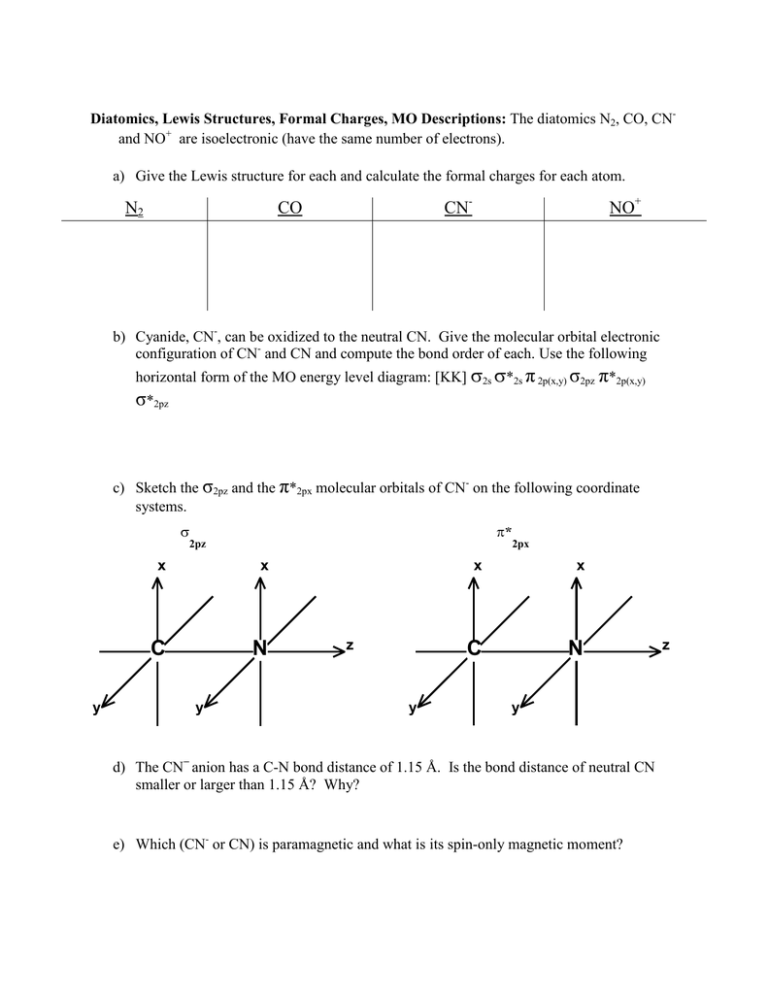
No+ mo diagram. Molecular Orbital Diagram of NO - All About Chemistry 1. 243. Molecular Orbital Diagram of NO. TAGS. Molecular Orbital Diagram. Previous article Molecular Orbital Diagram of CO. Next article Qualitative and Quantitative Analysis |Organic Chemistry. CHEM 2303 - Acadia U Heteronuclear Diatomic MO Diagrams. Question 1. Both the Lewis Structure and the MO Diagram predict three bonds and a lone pair of electrons on each atom. Since the HOMO is a bonding MO (and therefore based primarily on oxygen), NO + would be expected to coordinate through the oxygen atom. Molecular Orbitals of NO, NO+, and NO - ACS Publications by RP Orenha · 2014 · Cited by 13 — structure and molecular orbital theory of NO, NO+, and NO ... structure and the qualitative valence MO energy level diagram. What is the bond order of \[N{{O}^{+}}\] Let's see how much electrons are present in antibonding orbitals and bonding orbitals of N O + in order to determine the bond order by MO diagram. We can see that there are a total 2+2+2+2=8 electrons in the bonding orbitals. There are only 2 electrons in the antibonding orbitals as we have removed one electron from π ∗ antibonding orbital.
What is the bond order of NO and NO+? - BYJUS a = Number of electrons in bonding molecular orbitals. b = Number of electrons in antibonding molecular orbitals. (1) NO+ Total number of electrons in NO + = 14 Molecular configuration can be written as (σ1s) 2 (σ*1s) 2 (σ2s) 2 (σ*2s) 2 (π) 4 (2p z) 2 a = 10 b= 4 Bond order = 1/2 (10 - 4) Bond order = 1/2 (6) Bond order = 3 Bond order of NO+ is 3. MOT(VI)NO, NO+&NO- - YouTube ¤ Molecular Orbital diagram of BN, CN, CN- ¤Molecular Orbital diagram of COhttps://youtu.be/8mufOTgvagU¤ S-P mixing OF ORBITALS ... MO for HF - Chemistry LibreTexts A simple approach to molecular orbital (MO) theory for heterogeneous diatomic molecules is to show the energy level diagram. The MO energy levels can be worked out following these steps: Recall that the energy E n for the quantum number n is for an element with atomic Z is approximately. (1) E n = 13.6 Z E f f 2 n 2 e V. Draw the molecular energy level diagrams of ... - Study.com Draw the molecular energy level diagrams of NO + + , NO, and NO − − . Calculate their bond orders and give their magnetism (diamagnetic or paramagnetic). Bond Order: Bond order is the estimate of...
CHEMISTRY COMMUNITY - University of California, Los Angeles Molecular Orbital Diagram of NO+ Postby Josh Ku 3H » Tue Nov 15, 2016 7:23 pm viewtopic.php?f=43&t=16787 In the link above chem_mod said it was best to account for the negative charge of CN- by placing an extra electron on the nitrogen since it is more electronegative. I was just wondering if the same applied for molecules with a positive charge. Solution Manual Brady Chemistry 6TH Edition PDF | PDF ... Chapter 1. Practice Exercises 1.1 (a) SF6 contains 1 S and 6 F atoms per molecule (b) (C2H5)2N2H2 contains 4 C, 12 H, and 2 N per molecule (c) Ca3(PO4)2 contains 3 Ca, 2 P, and 8 O atoms per formula unit (d) Co(NO3)26H2O contains 1 Co, 2 N, 12 O, and 12 H per formula unit. 1.2 (a) NH4NO3 contains 2 N nitrogen, 4 H hydrogen, 3 O oxygen atoms per formula unit (b) … [Solved] (i) Construct a molecular orbital energy level ... So as we know bond order is inversely proportional to bond length, hence it can be depicted that NO+ have shorter bond length than Neutral NO . Ans -3 When NO+ is used as ligand it binds through N atom via sigma bonding by homo orbital 2pz and back bonding in its empty π*2p orbitals . Image transcriptions # Molecular orbital energy diagram of NO. Answered: Draw the M.O. diagram of NO+ . Indicate… | bartleby Science Chemistry Q&A Library Draw the M.O. diagram of NO+ . Indicate the HOMO, LUMO, and the expected bond order. Draw the M.O. diagram of NO+ . Indicate the HOMO, LUMO, and the expected bond order. Question. Draw the M.O. diagram of NO+ . Indicate the HOMO, LUMO, and the expected bond order.
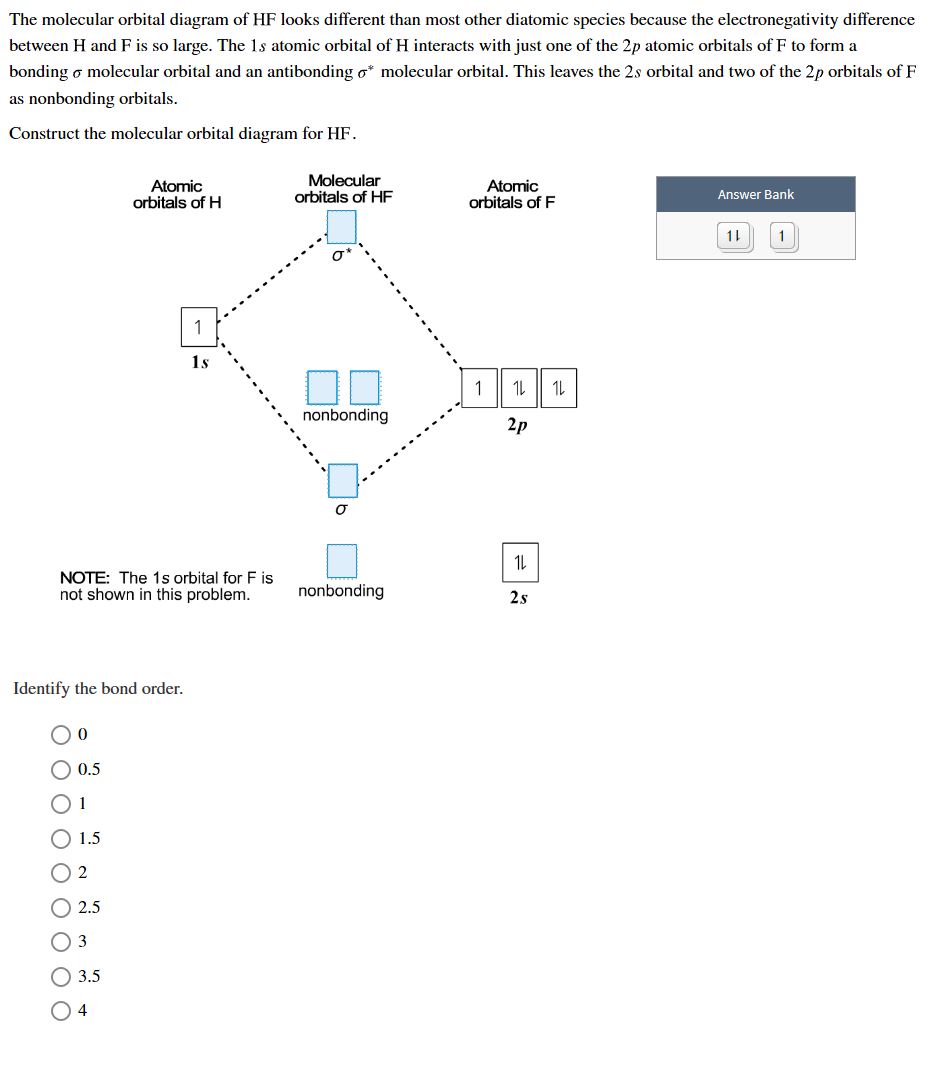
PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
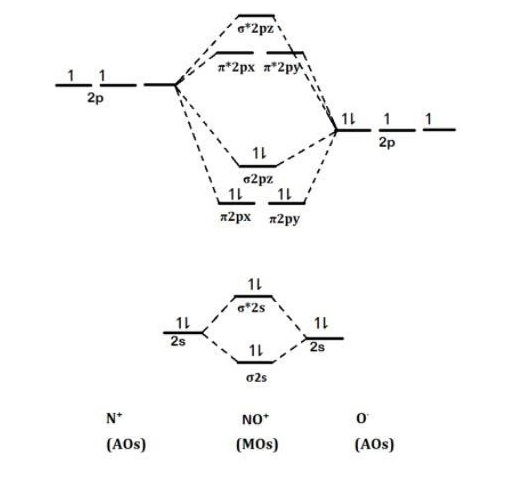
PDF Answer on the question #54656 ... - Assignment Expert The molecular orbital diagram for CO and NO+ molecule and ion are: The bond order is the difference between the number of the bonding electrons and the number of antibonding electrons, divided by two.
NO2 Lewis Structure, Molecular Geometry ... - Techiescientist Steric No = 2 sigma + 1 lone electron. The central N is less electronegative than O. Therefore, the charge on N is '+'. So, the oxidation state is found positive and the lone electron will take part in hybridization. steric no = 2 + 1 = 3. Therefore, the hybridization of NO2 is sp2. NO2 Polarity What is Polarity?
Solved: Draw the molecular orbital diagrams for NO- and ... 115E Draw the molecular orbital diagrams for NO - and NO +. Compare the bond orders in these two ions. Step-by-step solution Step 1 of 4 Molecular orbitals are formed by linear combination of atomic orbitals. Atomic orbitals and molecular orbitals of a molecule can be shown in a molecular orbital diagram. Chapter 10, Problem 115E is solved.
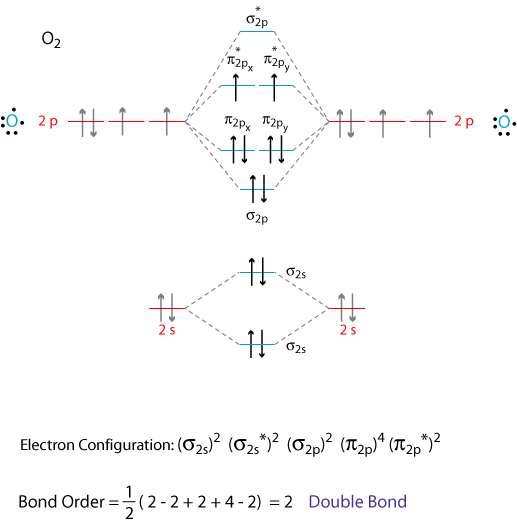
Explain the MO diagram for NO molecule. - Sarthaks Best answer 1. Electronic configuration of N atom is 1s2 2s2 2p3 2. Electronic configuration of O atom is 1s2 2s2 2p4 3. Electronic configuration of NO molecule is σ1s2 σ*1s2 σ2s2 σ*2s2 π2px2 π2py2 π2pz2 π*2px1 4. Bond order = N b−N a 2 N b − N a 2 = 10−5 2 10 − 5 2 = 2.5 5. NO molecule has one unpaired electron, hence it is paramagnetic
Consider the following molecules: NO, NO+ and NO ... - Quora Your MO diagram for NO should look like this: O is more electronegative than N so its orbitals are slightly lower in energy and the bonding orbitals are slightly more concentrated on O. Note the odd electron is in a Pi*2p orbital. Now draw two more MO diagrams for NO+ and NO-
Molecular Orbital Diagram Of NO,NO+ And HCl Molecules ... Lecture On Molecular Orbital Diagram Of NO,NO+ And HCl Molecules Which Is Explained In Details..Molecular Orbital Energy Level Diagramhttps://youtu.be/Go6VyX...
Order by bond length? "NO", "NO"^(+), "NO ... - Socratic.org From this MO diagram, we can see that: NO+ has 0 π* antibonding electrons. NO has 1 π* antibonding electron. NO− has 2 π* antibonding electrons. As the number of antibonding electrons increases, the N−O bond weakens, having acquired antibonding character (which as the name suggests, goes against making a bond).
Using the MO diagram of "NO", calculate the ... - Socratic.org Aug 29, 2017 · 4a1 is the σ* 2pz antibonding MO. To obtain the bond order, look at the molecular orbitals formed and decide whether they are bonding or antibonding. BO = 1 2 (bonding e− − antibonding e−) = 1 2 [(2 + 2 + 2 + 2) − (2 + 1)] = 2.5. And this should make sense because NO+ is isoelectronic with CO, which has a bond order of 3.
The nitrosyl ion, NO+, has ten bonding electrons and ... The nitrosyl ion, NO+, has ten bonding electrons and four antibonding electrons. Draw the MO energy diagram fo this species. Use this diagram todetermine the bond order of NO. Question: The nitrosyl ion, NO+, has ten bonding electrons and four antibonding electrons. Draw the MO energy diagram fo this species.
Bef2 Molecular Orbital Diagram An incomplete MO diagram for NO+ is provided. a. (6 pts.) The point group for BeF2 is D∞h, but when determining the symmetry of the group orbitals .The linear combination of atomic orbitals always gives back the same number of molecular orbitals. So if we start with two atomic orbitals (e.g., an s and a p z orbital as shown in Fig. ), we end ...
Draw the molecular orbital diagrams for NO, NO+, and NO-. For each molecule, determine the bond order, if the molecule is stable, and if the molecule is stable if it is paramagnetic or diamagnetic. Rank the molecules in increasing order of bond strength.
Cyanide Molecular Orbital Diagram - schematron.org A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. In this answer of Martin's, you can find a molecular orbital diagram of $\ce {CO}$.
energy - Chemistry Stack Exchange For this reason, I am reluctant to present a molecular orbital diagram. However, I will provide calculated MOs. While it may be tempting to just look at the pictures, the rationalisation behind it is more important. What will follow now are a few attempts at that rationalisation. In the nitric oxide series, $\ce{NO+}$ is surprisingly the simplest.
Chemistry Community Nov 21, 2011 · Re: MO diagram for NO+. Postby Chem_Mod » Mon Nov 21, 2011 11:19 pm. Just draw the atomic orbitals of O lower. This will result in the antibonding molecular orbitals being closer to N. Top. 2 posts • Page 1 of 1. Return to “*Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)”. Jump to.
NO, NO+ and NO-. Using the molecular orbital theory, how ... Your MO diagram for NO should look like this: O is more electronegative than N so its orbitals are slightly lower in energy and the bonding orbitals are slightly more concentrated on O. Note the odd electron is in a Pi*2p orbital. Now draw two more MO diagrams for NO+ and NO-





















0 Response to "40 no+ mo diagram"
Post a Comment