36 orbital diagram for scandium
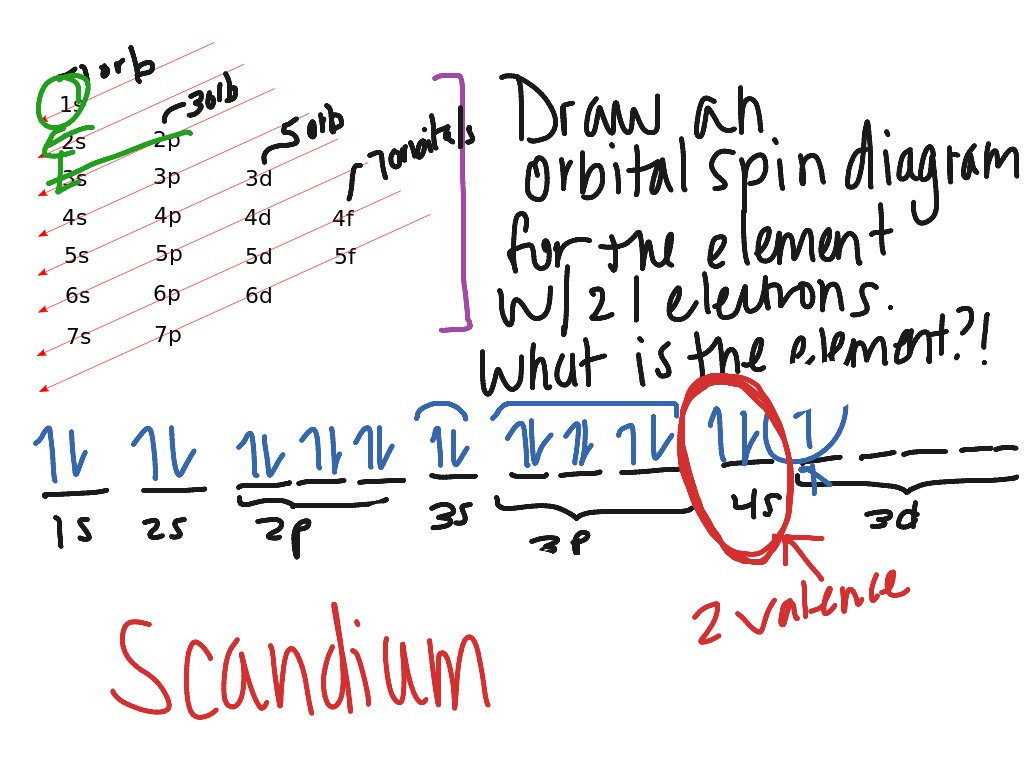
Orbital Diagram For Scandium - Wiring Diagrams An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. Scandium has an atomic no. of Therefore electronic configuration of scandium (Sc) is: 1s2 2s2 2p6 3s2 3p6 3d1 4s2. Here, two electrons are in 4s orbital. wiringall.com! Solved Fill in the orbital energy diagram for scandium. 3d ... Expert Answer 100% (2 ratings) Scandium (Sc) is the first element in … View the full answer Transcribed image text: Fill in the orbital energy diagram for scandium. 3d 3p E 35 AV AV AV 2p NV 2s AV 1s The lowest Elevels are already filled in for you Previous question Next question
› topics › medicine-andX-Ray Tube - an overview | ScienceDirect Topics X-rays and gamma rays are electromagnetic radiations. • X-rays are produced by stopping high-energy electrons in a tungsten target. • X-rays are emitted in a continuous spectrum (bremsstrahlung) with a maximum energy equal to the peak accelerating potential of the X-ray tube and at discrete energies dependent on the binding energies of electrons in the target atom.

Orbital diagram for scandium
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital Diagram For Vanadium (V) | Vanadium Electron ... The symbol of the element Vanadium is represented by "V" and the Vanadium Electron Configuration is given as below; Ar 3d3 4s2. To make it, even more, easier you can refer to the image and clear your doubts. Vanadium Number of Valence Electrons What is the orbital diagram for scandium What is the orbital diagram for scandium. Answers: 1 Show answers Another question on Chemistry. Chemistry, 21.06.2019 20:00. Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c? Answers: 1. Answer. Chemistry, 22.06.2019 13:00. Lab reagent, hypothesis test.a reference solution used as a lab ...
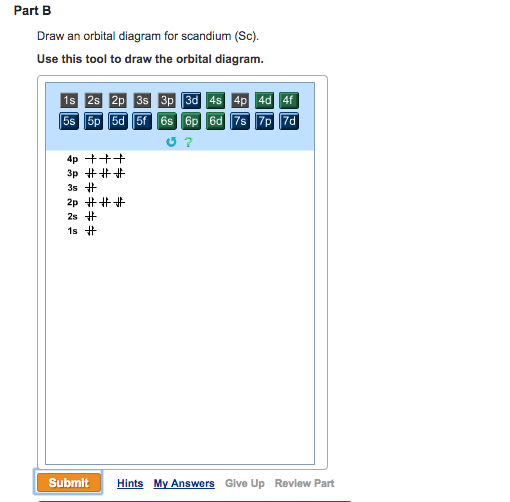
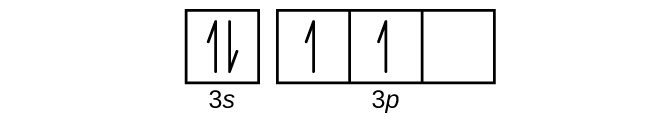
Orbital diagram for scandium. PDF Electron configuration and orbital diagram worksheet For example, write the electron configuration of scandium, sc:1s2 2s2 2p6 3s2 3p6 4s2 3d1 . So for the 1st scandium and 2nd electrons must be in orbital 1s, the 3rd and 4th of the 2s, the 5th to the 10th of the 2p orbital, etc. This is a memory device to remember the order of orbital for the first two quantity numbers. Draw An Orbital Diagram For Scandium (sc) How do you draw an orbital diagram for scandium (Sc)? electron residing in the 3d orbital is found to be very unstable, Hence Sc Readily loses an electron to . Answer to Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. How to Write the Atomic Orbital Diagram for Scandium (Sc ... To write the orbital diagram for the Scandium atom (Sc) first we need to write the electron configuration for just Sc. To do that we need to find the number ... Draw an orbital diagram for scandium (Sc). | Study.com An orbital diagram is the representation of electrons present in different orbitals. There are four orbitals, s-orbital, p-orbital, d-orbital, and f-orbital.1 answer · Top answer: Orbital diagram of Scandium The atomic number of Sc is 21. The electronic configuration of Sc is...
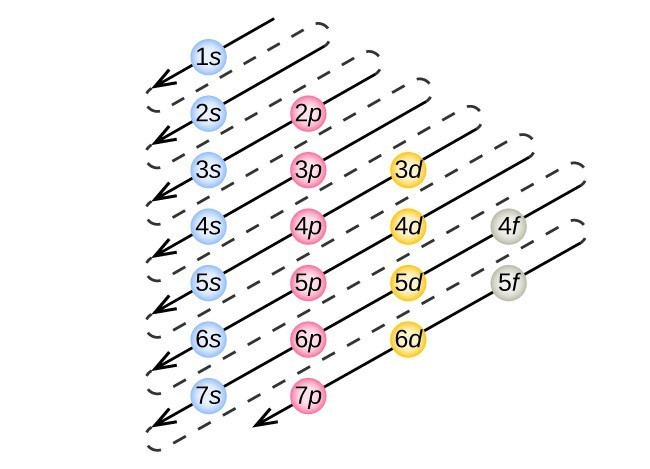
PDF Electron orbital notation scandium So for scandium the 1st and 2nd electron must be in 1s orbital, the 3rd and 4th in the 2s, the 5th through 10th in the 2p orbitals, etc. This is a memory device to remember the order of orbitals for the first two quantum numbers. Follow the arrow starting in the upper right, when the arrow ends go to the next arrow and start again. orbital diagram for scandium - Brainly.in 16 Oct 2020 — The atomic number of Sc is 21 which means Sc has 21 electrons. The location of Sc in the periodic table is shown below: Based on the figure, Sc ...1 answer · 5 votes: Answer:The atomic number of Sc is 21 which means Sc has 21 electrons. The location of Sc in the periodic table is shown below: Based on the figure, Sc ... PDF Electron Configurations and Orbital Diagrams key Draw orbital diagrams for the following elements. Write the electron configuration (full, and in core notation): 1. scandium ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑ 1s 2s 2p 3s 3p 4s 3d Full electron configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1 Core notation: [Ar] 4s 2 3d 1 2. Electron configuration for Scandium (element 21). Orbital ... Sc (Scandium) is an element with position number 21 in the periodic table. Located in the IV period. Melting point: 1539 ℃. Density: 2.99 g/cm 3 . Electronic configuration of the Scandium atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1. Electronic configuration of the Scandium atom in ascending order of the levels:
How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. en.wikipedia.org › wiki › NitrogenNitrogen - Wikipedia Its bonding is similar to that in nitrogen, but one extra electron is added to a π* antibonding orbital and thus the bond order has been reduced to approximately 2.5; hence dimerisation to O=N–N=O is unfavourable except below the boiling point (where the cis isomer is more stable) because it does not actually increase the total bond order ... Phosphorus(P) electron configuration and orbital diagram Phosphorus orbital diagram According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. The 1s orbital is now filled with two electrons. The next two electrons will enter the 2s orbital just like the 1s orbital. Scandium(Sc) electron configuration and orbital diagram Scandium (Sc) excited state electron configuration and orbital diagram When scandium atoms are excited, then scandium atoms absorb energy. As a result, an electron in the 4s orbital jumps to the 4px sub-orbital. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z. Each sub-orbital can have a maximum of two electrons.
PDF Electron configuration and orbital diagram for scandium So for scandium the 1st and 2nd electron must be in 1s orbital, the 3rd and 4th in the 2s, the 5th through 10th in the 2p orbitals, etc. This is a memory device to remember the order of orbitals for the first two quantum numbers. Follow the arrow starting in the upper right, when the arrow ends go to the next arrow and start again.
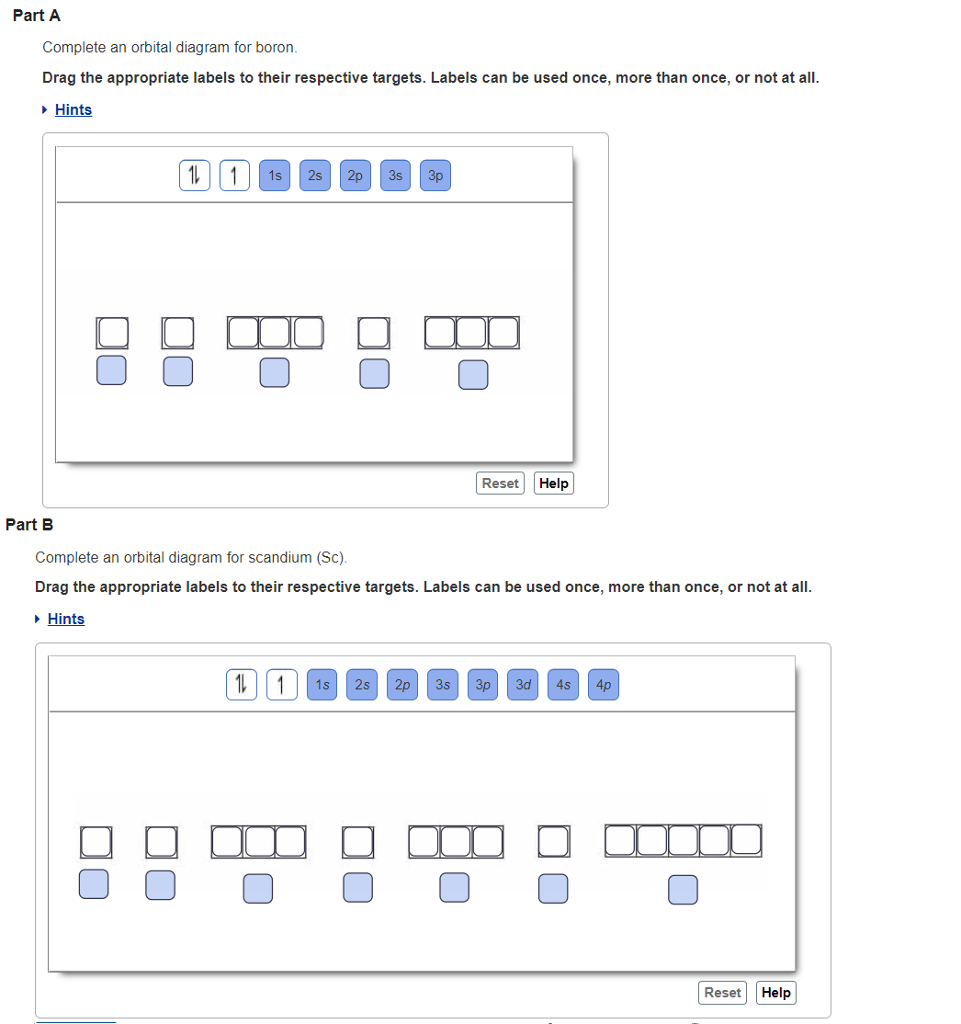
Draw An Orbital Diagram For Boron. Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. How many orbitals are there in the third shell (n = 3)? Express your answer numerically as an integer. Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest ...
Draw an orbital diagram for scandium Sc? - Answers Orbital diagram for Sc? the electronic configuration for sc is 1s2 2s2 2p6 3s2 3p6 4s2 3d1 Is scandium Sc a metal? Scandium is a transition metal. What is the formula for scandium III and iodate...
chem 1201 HW Ch. 6 p 2 Flashcards | Quizlet from an orbit of radius = 4.76 Å to one of radius 0.529 Å energy is absorbed: from the n=6 to the n=9 state from the n=2 to the n=5 state Using equation E= (hcRH) (1n2)= (−2.18×10−18J) (1n2), calculate the energy of an electron in the hydrogen atom when n=2. E2 = −5.45×10−19 J
PDF Ground state electron configuration for scandium In addition to listing the main quantum number, n, and subsella, \ (\ell\), the orbital diagram shows all the different orientations and spins of each electron. The diagram shows the number of subshells using boxes or lines for electrons (used three for the orbital p, five for the orbital d and 7 for the orbital f).
Draw an orbital diagram for scandium (Sc)? - ForNoob Orbital Diagram For Scandium. draw orbital diagram scandium sc. Discover The Secrets Of Drawing Realistic Pencil Portraits. This will help you to achieve mastery in a very short period of time. All of these break down into 5 lessons of realistic facial features drawing.
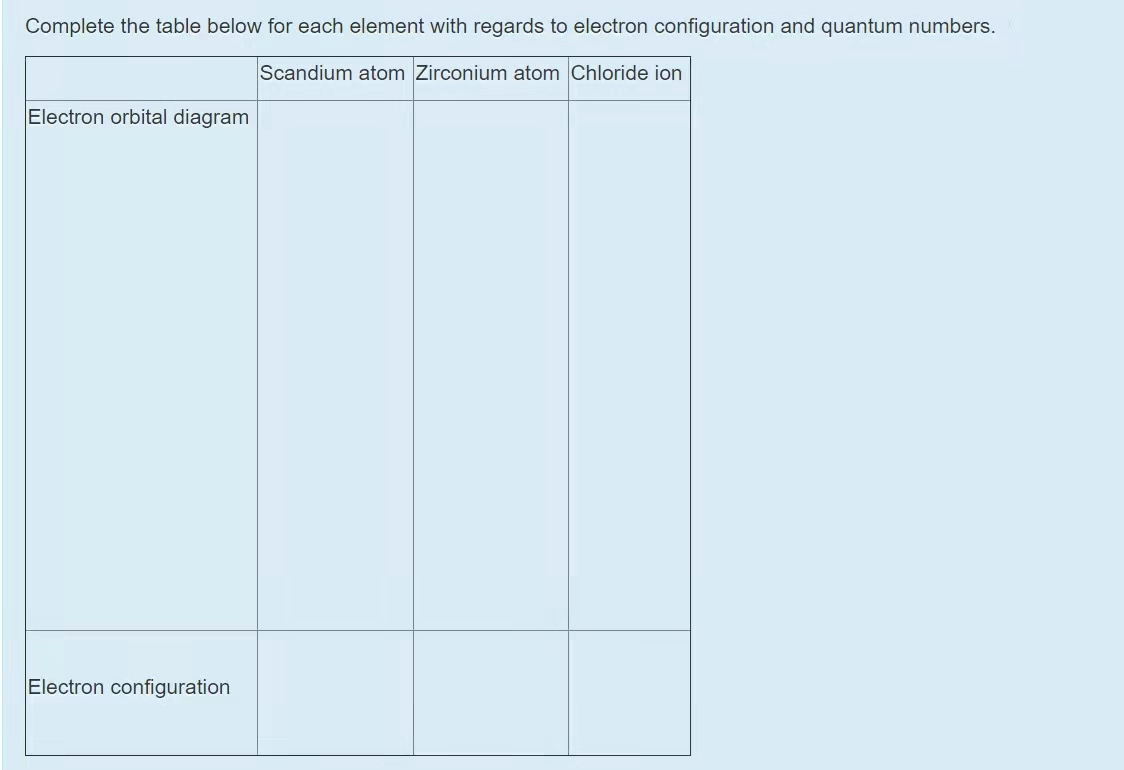
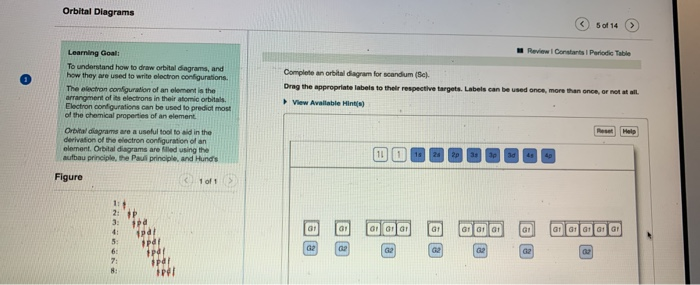
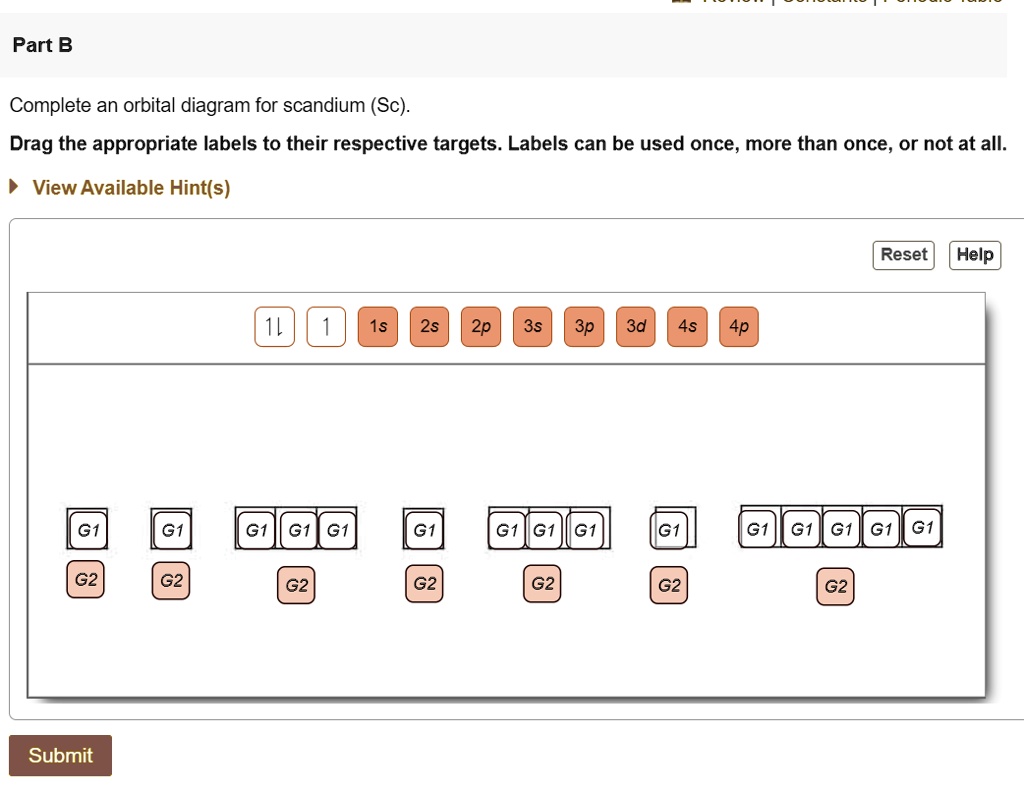
Complete An Orbital Diagram For Scandium (sc). Answer to Part B Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be used. Contrary to what you may have seen, for Sc and the remaining elements, the 4s is not lower in energy than the 3d. 21- Scandium electronic configuration with translucent orbitals In fact, for elements with. 3d.
PDF Sc orbital diagram Complete an orbital diagram for scandium (Sc). you must draw an electon in boxes diagram the full configuration is: 1s2 2s2 2p6 3s2 3p6 4s2 3d1 s orbital has 1 box (squares) p orbital has 3 boxes d orbital has 5 boxes in each box fill it with arrows (up and down Scandium is a chemical element with the symbol Sc and atomic number 21.
Complete An Orbital Diagram For Scandium (sc). Draw orbital diagrams for the following elements. Write the electron configuration (full, and in core notation). 1. scandium. ↑↓. ↑↓. ↑↓ ↑↓ ↑↓. ↑↓. Contrary to what you may have seen, for Sc and the remaining elements, the 4s is not lower in energy than the 3d. In fact, for elements with.
What is the orbital diagram for scandium What is the orbital diagram for scandium. Answers: 1 Show answers Another question on Chemistry. Chemistry, 21.06.2019 20:00. Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c? Answers: 1. Answer. Chemistry, 22.06.2019 13:00. Lab reagent, hypothesis test.a reference solution used as a lab ...
Orbital Diagram For Vanadium (V) | Vanadium Electron ... The symbol of the element Vanadium is represented by "V" and the Vanadium Electron Configuration is given as below; Ar 3d3 4s2. To make it, even more, easier you can refer to the image and clear your doubts. Vanadium Number of Valence Electrons
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30:






















0 Response to "36 orbital diagram for scandium"
Post a Comment