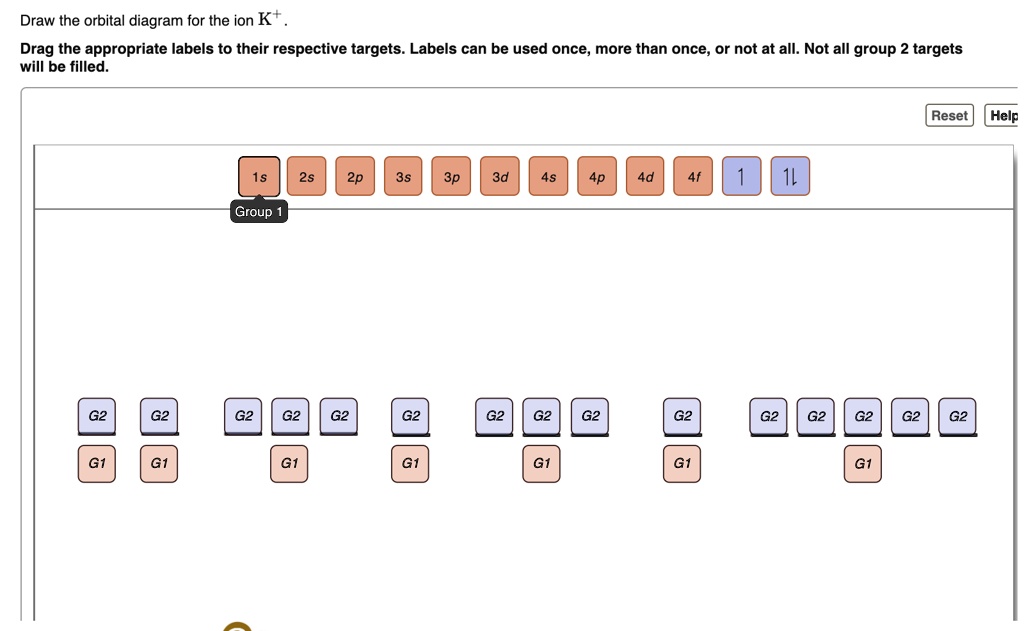
39 orbital diagram for k
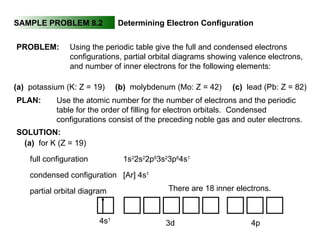
Solved 6. What is full and condensed electron ... (10 pts) Element, K 19) Your answer Full electron configuration Condensed electron configuration Partial orbital diagram 7. What are the Lewis electron-dot symbol for the following elements? (10 pts) Lewis electron-dot symbol Element Lewis electron-dot symbol Element Mg Na. Krypton Orbital Diagram Feb 08, 2018 · Orbital Diagram. 1s. ↿⇂. 2s. ↿⇂. Black area in the center as well as the 4s and 4p orbitals with white electron arrows are orbitals which are included in krypton and are already full. Since krypton is in the far right row of the periodic table, its outermost shell is full with eight electrons.
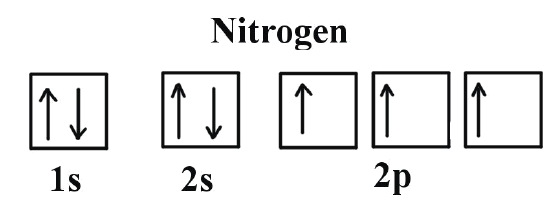
Orbital Diagram For Nitrogen (N) | Nitrogen Electron ... Orbital Diagram For Nitrogen (N) | Nitrogen Electron Configuration February 15, 2021 by Sneha Leave a Comment Nitrogen Electron Configuration : When we talk about school subjects, then one of the major subjects which are very important for knowledge perspective is science .

Orbital diagram for k
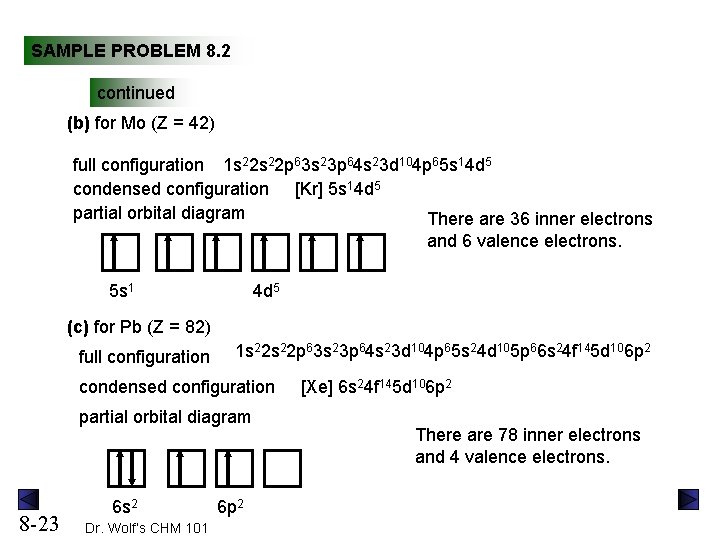
Electron configuration for Lead (element 82). Orbital diagram Reduced electronic configuration Pb: [Xe] 4f 14 5d 10 6s 2 6p 2. Below is the electronic diagram of the Lead atom Distribution of electrons over energy levels in the Pb atom. 1-st level (K): 2. 2-st level (L): 8. 3-st level (M): 18. 4-st level (N): 32. Orbital Diagrams - KTharpeSaaChemistry Chapter 11: Modern Atomic Theory. Orbital Diagrams. Back to Chapter 11. Orbital Diagrams. Orbital (box) Diagram: Orbitals are represented by boxes grouped by sub levels (subdivision of the principal energy level) with small rows indicating the the electrons. How to Write the Orbital Diagram for Potassium (K) - YouTube To write the orbital diagram for the Potassium atom (K) first we need to write the electron configuration for just K. To do that we need to find the number o...
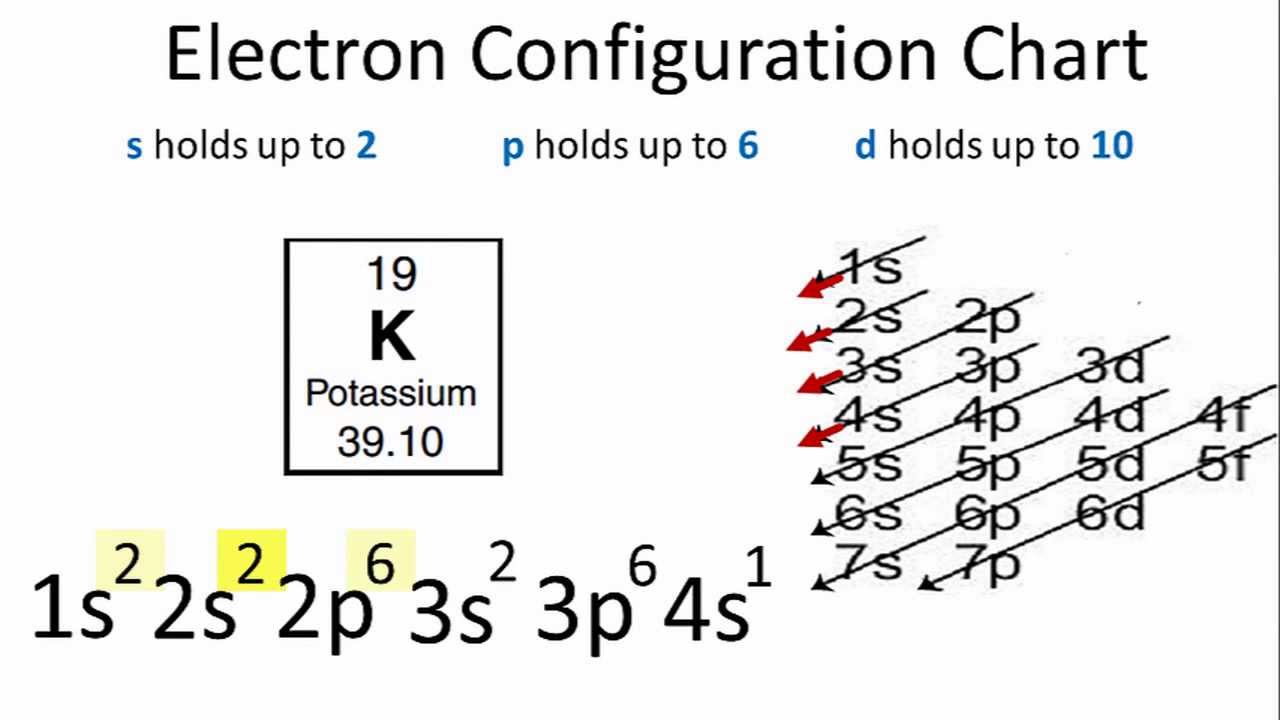
Orbital diagram for k. Electron Configuration for Potassium (K) When we write the configuration we'll put all 19 electrons in orbitals around the nucleus of the Potassium atom. In writing the electron configuration for Potassium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Potassium go in the 2s orbital. PDF Chemistry: Orbital Diagrams Chemistry: Orbital Diagrams Using forward slashes ( / ) and backslashes ( \ ), construct the orbital diagram for each of the following elements. Orbitals… Element 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p H Li Na K Rb Be Mg Ca Sr C O S F Cl Br I He Ne Ar Kr Xe Fe Potassium(K) electron configuration and orbital diagram To write the orbital diagram of potassium (K), you have to do the electron configuration of potassium. Which has been discussed in detail above. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. Potassium orbital diagram PDF Orbital Diagram Lab - New Providence School District Orbital Diagram Lab Background The electrons in an atom occupy distinct principal energy levels. To be located in any each of these principal energy levels, electrons must have the required energy for the level. The principal energy levels are numbered 1, 2, 3, 4, 5, 6, and 7for the atoms of the known elements.
Potassium (K) Orbital diagram, Electron configuration, and ... Potassium (K) Orbital diagram, Electron configuration, and Valence electrons Find this Pin and more on Chemistry sectionby Vishal Goyal. More like this Potassium Nitrate Molecular Shapes Inorganic Compound Ammonium Hydroxide Is Water (H2O) an acid or base or neutral? - It's Conjugate acid and Conjugate base Potassium (K) - ChemicalAid Description Soft, waxy, silver-white metal. Eighth most abundant element in the earth's crust (20,900 ppm). Occurs only in compounds. Uses Used as potash in making glass & soap. Also as saltpeter, potassium nitrate (KNO3) to make explosives and to color fireworks in mauve. Formerly called kalium (K). Vital to function of nerve and muscle tissures. PDF Orbitals, and the Periodic Table - UC Santa Barbara The energy of an electron in an orbital with quantum number nfor an atom with atomic number Zis given by: E n = ... There is a simple way of remembering how electrons fill up orbitals, shown in the accompanying diagrams: 6d 1s 2s 3s 4s 5s 6s 2p 3p 4p 5p 6p 3d 4d 5d 4f 5f 2. Materials 100A: Orbitals, bonding, etc. 19 20 K Ca 1 2 H He 3 4 Li Be ... Iridium(Ir) electron configuration and orbital diagram Iridium (Ir) electron configuration and orbital diagram. Iridium is the 77th element in the periodic table and its symbol is 'Ir'. Iridium is a classified transition metal element. The total number of electrons in iridium is seventy-seven. These electrons are arranged according to specific rules of different orbits.
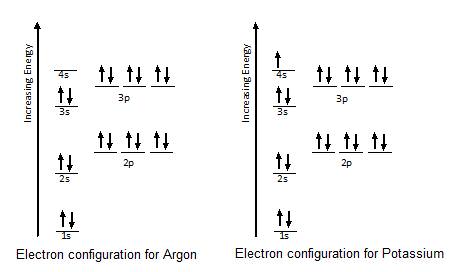
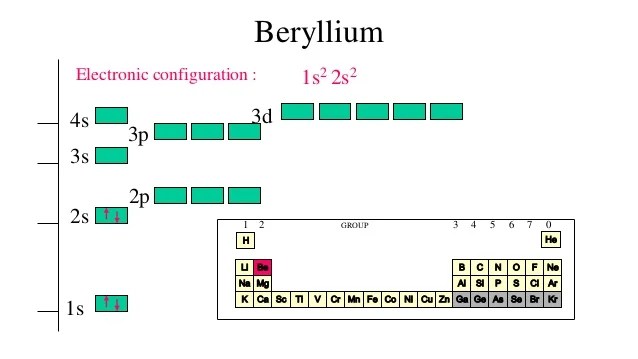
Orbital Diagrams - Concept - Chemistry Video by Brightstorm Orbital diagrams are a pictorial description of electrons in an atom. In order to figure out where electrons go in an atom we have to follow 3 main rules. The first one being the Auf Bau Principle, the Auf Bau Principle states that each electron occupies the lowest energy orbital available. Then we have to think okay with the sublevels, I mean ... Beryllium Orbital Diagram - schematron.org A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. Argon Orbital diagram, Electron configuration, and Valence ... The orbital diagram simply represents the arrangement of electrons in the different orbitals of an atom, it uses an arrow to represent the electrons, every orbital (one box) contains a maximum of 2 electrons. There are three rules followed for constructing the orbital diagram for an atom. (1). Atomic orbital - Wikipedia In atomic theory and quantum mechanics, an atomic orbital is a mathematical function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus.The term atomic orbital may also refer to the physical region or space where the electron can be ...
Solved Draw the orbital energy diagram for K (potassium ... Question: Draw the orbital energy diagram for K (potassium). Represent electrons as arrows (with up or down spin). What is the core] valence electron configuration? (type in the box below) -45 Energy 2p 2s is *** -T-5 This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (2 ratings)
Draw An Orbital Diagram For Boron. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.Electron Configuration for Boron (B)Electron Configuration for Boron (B)
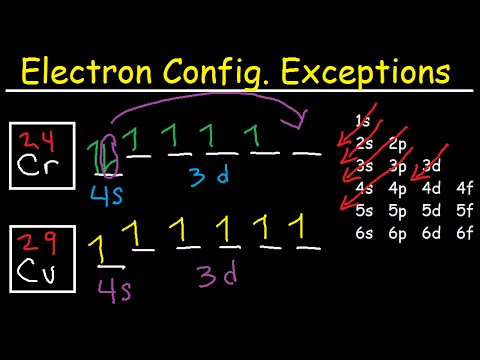
Electron configuration for Chromium (element 24). Orbital ... Orbital diagram Chromium electron configuration ← Electronic configurations of elements The order of filling the orbitals with electrons in the Cr atom is an exception to the rule. Expected electronic configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d4 But in reality, one electron moves from the 4s orbital to the 3d orbital:
Orbital Diagrams and Electron Configuration - Basic ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram notation (with arrows) of an element given...
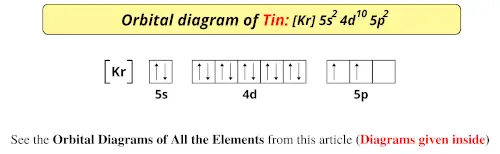
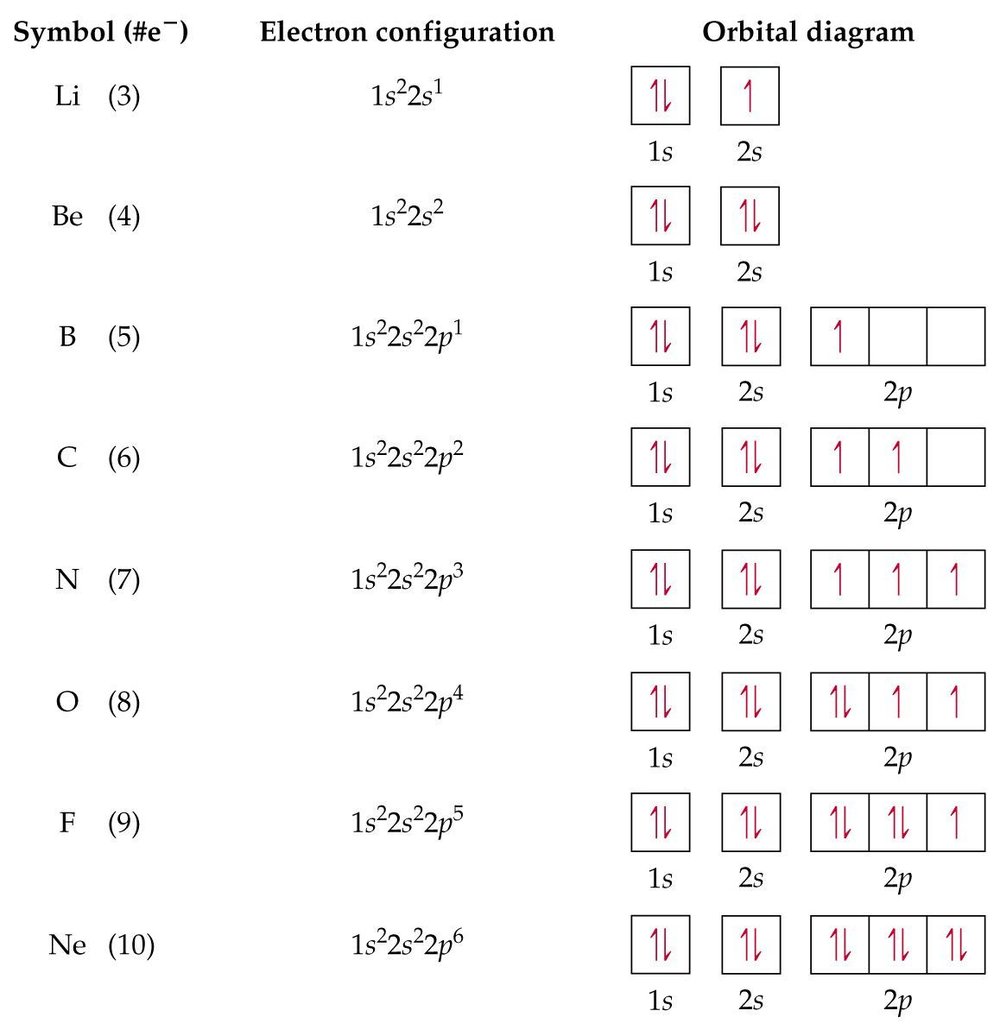
Orbital Diagram of All Elements (Diagrams given Inside) Apr 10, 2021 · Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).
Potassium (K) Orbital diagram, Electron configuration, and ... The Potassium orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, the six electrons in the 2p orbital, the two electrons in the 3s orbital, the next six electrons in the 3p orbital, and the remaining one electron will go in 4s orbital. An orbital diagram for a ground-state electron configuration of a Potassium ...
What is the orbital notation of k? - Answers Orbital Notation is a way to show how many electrons are in an orbital for a given element. What is the orbital notation for Mn? The notation would be 1s22s22p63s23p64s23d5, or in noble gas ...
Mo3+ Orbital Diagram - schematron.org Mo3+ Orbital Diagram. That's why Mo3+ has 39 electrons instead of 42 in its ground state electron The normal electron configuration of zinc is [Ar] 3d 10 4s 2, with 2 4s orbital. However, even though the 5s orbital is lower in energy than the 4d orbital, the electrons in the 4d orbitals shield the electron in the 5s orbitals.
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
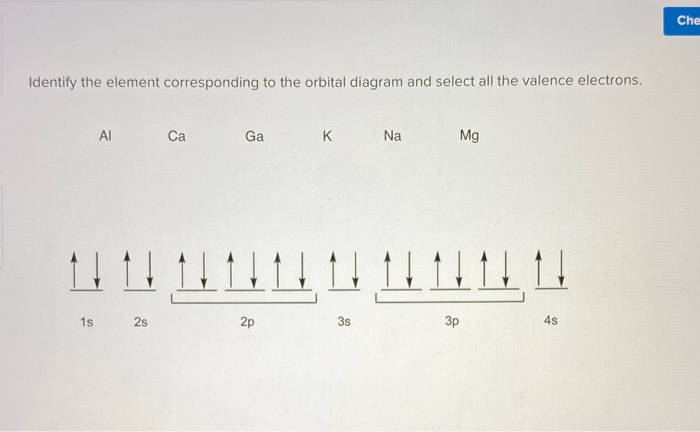
PDF Electron Configurations and Orbital Diagrams key 1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals?
How to Write the Orbital Diagram for Potassium (K) - YouTube To write the orbital diagram for the Potassium atom (K) first we need to write the electron configuration for just K. To do that we need to find the number o...
Orbital Diagrams - KTharpeSaaChemistry Chapter 11: Modern Atomic Theory. Orbital Diagrams. Back to Chapter 11. Orbital Diagrams. Orbital (box) Diagram: Orbitals are represented by boxes grouped by sub levels (subdivision of the principal energy level) with small rows indicating the the electrons.
Electron configuration for Lead (element 82). Orbital diagram Reduced electronic configuration Pb: [Xe] 4f 14 5d 10 6s 2 6p 2. Below is the electronic diagram of the Lead atom Distribution of electrons over energy levels in the Pb atom. 1-st level (K): 2. 2-st level (L): 8. 3-st level (M): 18. 4-st level (N): 32.




















0 Response to "39 orbital diagram for k"
Post a Comment