37 democritus atom model diagram
study.com › academy › lessonEarly Atomic Theory: Dalton, Thomson, Rutherford ... - Study.com Aug 22, 2021 · A diagram of the Rutherford alpha particle experiment ... including Aristotle and Democritus' opposing views of the atom - Aristotle believing matter could be divided forever, and Democritus ... Chemistry, more like cheMYSTERY to me! - Democritus Democritus's Atomic Theory is the foundation for all of chemistry and is incredibly relevant today. This is where the chemistry Modeling Instruction curriculum starts. Democritus made the observationthat if you break a rock into tiny pieces, those pieces are still made of rock.
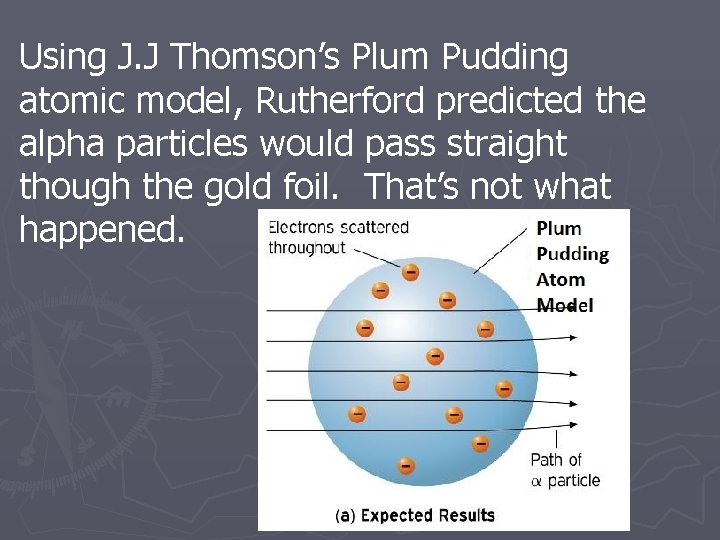
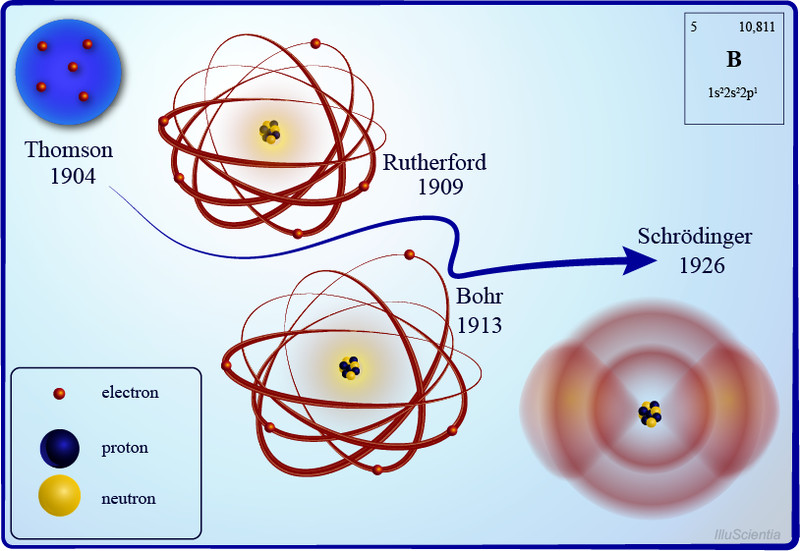
Rutherford Model of the Atom: Definition & Diagram The Rutherford model of the atom is one of the most popular representations of the atom. Explore the definition, diagram, development, and problems of the Rutherford model, as well as the ...

Democritus atom model diagram
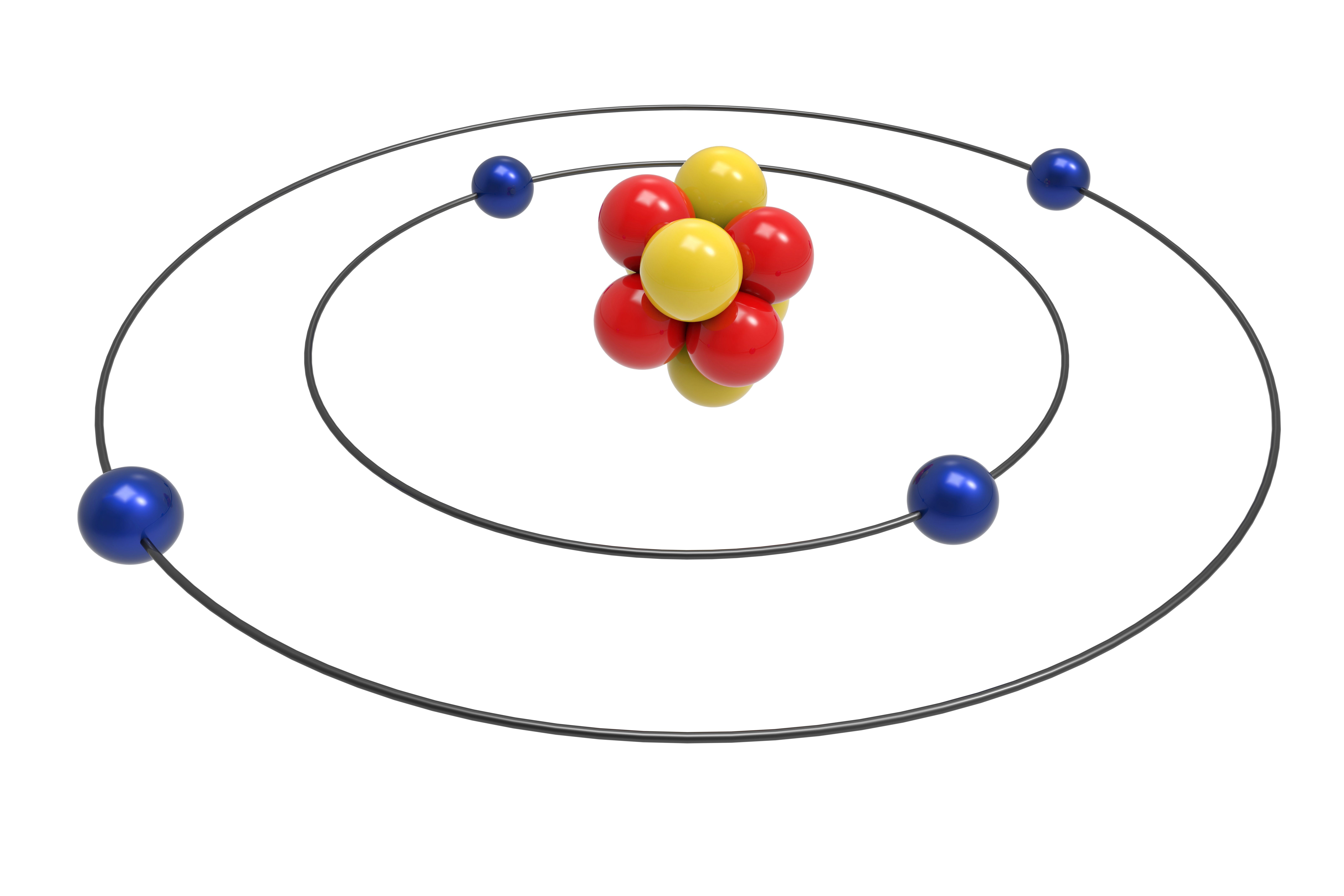
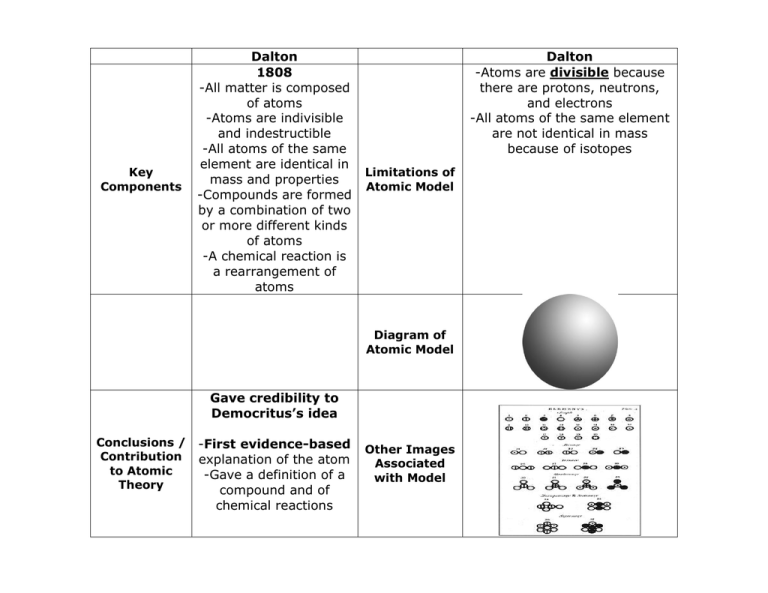
democritus Flashcards and Study Sets | Quizlet J. J. Thomson. Robert Milikan (1909) First to propose that matter was made up of invisible particle…. All matter is made up of atoms (Solid Sphere Model) 1897 used cathode ray and positively charged plates to ( (disco…. Oil Drop Experiment; tiny oil droplets on metal plate with hol…. Democritus. Dalton's Model of the Atom and Early Atomic Theory Atoms are small, chemically indestructible particles of matter. Elements consist of atoms. Atoms of an element share common properties. Atoms of different elements have different properties and different atomic weights. Atoms that interact with each other obey the Law of Conservation of Mass. Essentially, this law states the number and kinds of atoms that react are equal to the number and kinds of atoms in the products of a chemical reaction. The Structure of an Atom Explained With a Labeled Diagram The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge.
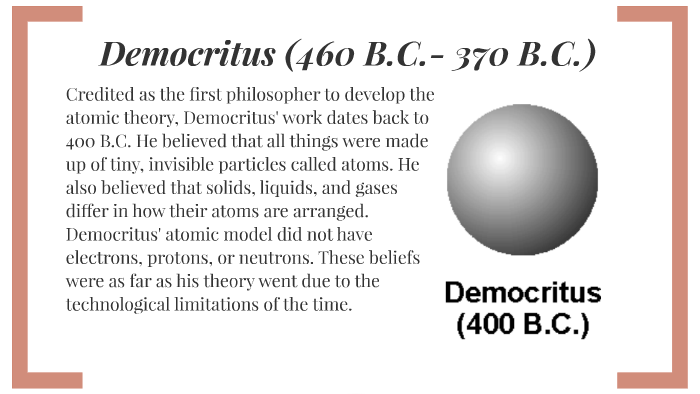
Democritus atom model diagram. Atomic Theory Project timeline | Timetoast timelines Democritus believed everything was made of of a very small particles called 'atomos'.And these atomos cannot be divided. He believed this because he thought if you get a stone and keep breaking it, at some point you cant break it any more.Fun Fact: Democritus believed atom vary in size and shape. Thomson Atomic Model - Plum pudding model, Postulates ... Thomson atomic model was proposed by William Thomson in the year 1900. This model explained the description of an inner structure of the atom theoretically. It was strongly supported by Sir Joseph Thomson, who had discovered the electron earlier. During cathode ray tube experiment, a negatively charged particle was discovered by J.J. Thomson. Basic Model of the Atom - Atomic Theory - ThoughtCo The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). Protons and neutrons form the atomic nucleus. Electrons are attracted to the protons in the nucleus, but are moving so quickly they fall toward it (orbit) rather than stick to protons. › uploads › 4/1/0Grade 9 Science Unit 1: Atoms, Elements, and Compounds INSIDE THE ATOM • An atom is the smallest particle of an element that retains the properties of the element. • All atoms are made up of three kinds of smaller particles call subatomic particles. • Three Subatomic Particles: Protons (positively charged) Electrons (negatively charged) Neutrons (no charge)
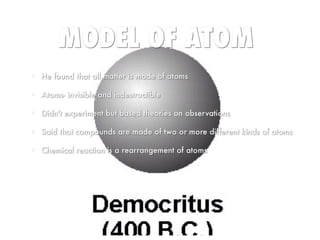
Democritus Atom Model Diagram Democritus Atom Model Diagram 15.08.2018 2 Comments The idea of atoms was invented by two Greek philosophers, Democritus and Leucippus in the fifth century BC. The Greek word ατoμoν (atom) means indivisible. B.C. - Democritus thought matter could not be John Dalton. • -Dalton proposed a modern atomic model Bohr - Rutherford diagrams. • Putting all. The History of the Atom - Home Democritus; John Dalton; J.J. Thomson; Lord Rutherford; Niels Bohr; Modern Quantum Model: Schrodinger and Chadwick; Atomic Theories Comparison; Your Turn: Candy Model? democritus atomic model Flashcards and Study Sets | Quizlet Browse 500 sets of democritus atomic model flashcards. Study sets. Diagrams. Classes. Users. Options. 6 terms. arianexcc. Early models of the atom: Democritus and Dalton's theory. Atomic Model of Democritus theory - Thethoughtnow One of the first atomic theorists was Democritus, a Greek philosopher who lived in the 5th century BC. Democritus knew that if a stone was divided in half, the two halves would have essentially the same properties as the whole. Therefore, he reasoned that if the stone were cut continuously into smaller and smaller pieces; At some point, there would ...
› 39605905 › Cambridge_CheckpointCambridge Checkpoint Science Coursebook 8 - Academia.edu This captivating Coursebook provides coverage of stage 8 of the revised Cambridge Secondary 1 curriculum framework. It is endorsed by Cambridge International Examinations for use with their programme. The series is written by a highly experienced Atomic Model: Rutherford, Bohr, Dalton, Thomson - Embibe Q.3. What are the five atomic models? Ans: The five atom is models are as follows: John Dalton's atomic model J.J Thomson's atomic model- Plum pudding model Ernest Rutherford model- Nuclear model of an atom Neil Bohr's model of the atom- Planetary model Erwin Schrödinger's model-Quantum model. Q.4. What is the most accurate atomic model? Difference Between Democritus and Dalton Atomic Theory Democritus atomic theory is the ancient theory that describes the nature of matter in terms of atoms. According to Democritus (99-55 BC), atoms are infinite in number and eternal. Figure 01: Democritus We cannot create them, and the composition of atoms in a substance determines the qualities of that substance. Democritus - The History of the Atom Description of his model: Democritus's model stated that matter consists of invisible particles called atoms and a void (empty space). He stated that atoms are indestructible and unchangeable. Also that they are homogenous, meaning they have no internal structure.
Democritus atom diagram? - Answers Democritus was not discovered the atom; he proposed and accepted intutively the notion of atom at approx. 400 bc. 2. Now the founder of atomism is considered Leucippus, precursor and teacher of ...
Democritus Atom Model Diagram - Wiring Diagrams Democritus Atom Model Diagram. Democritus was an Ancient Greek pre-Socratic philosopher primarily remembered today for his formulation of an atomic. This is due to his theory of universe that is made up of tiny "atoms", which bears .. Democritus' model of an atom was one of an intert solid that. In this lesson, we will review the development of the ...
5 Democritus Theory of Atoms - Structure - Model ... Democritus was not able to describe atomic model in detail. On his theory, Democritus only stated that atoms are in the solid form in the void sphare. We can not describe the internal structure of the atom itself. We now know that Atoms consist of 3 parts which are proton, neutron and electron. 2.
Atomic model of Democritus: antecedents, characteristics ... Democritus' atom: a long-forgotten model. For Aristotle the atomism of Democritus contradicted the concept of substance, in which the proportion of the elements (earth, air, water and fire) had to be maintained at all costs, no matter how small the fraction of it was. The substance for Aristotle is intrinsically continuous.
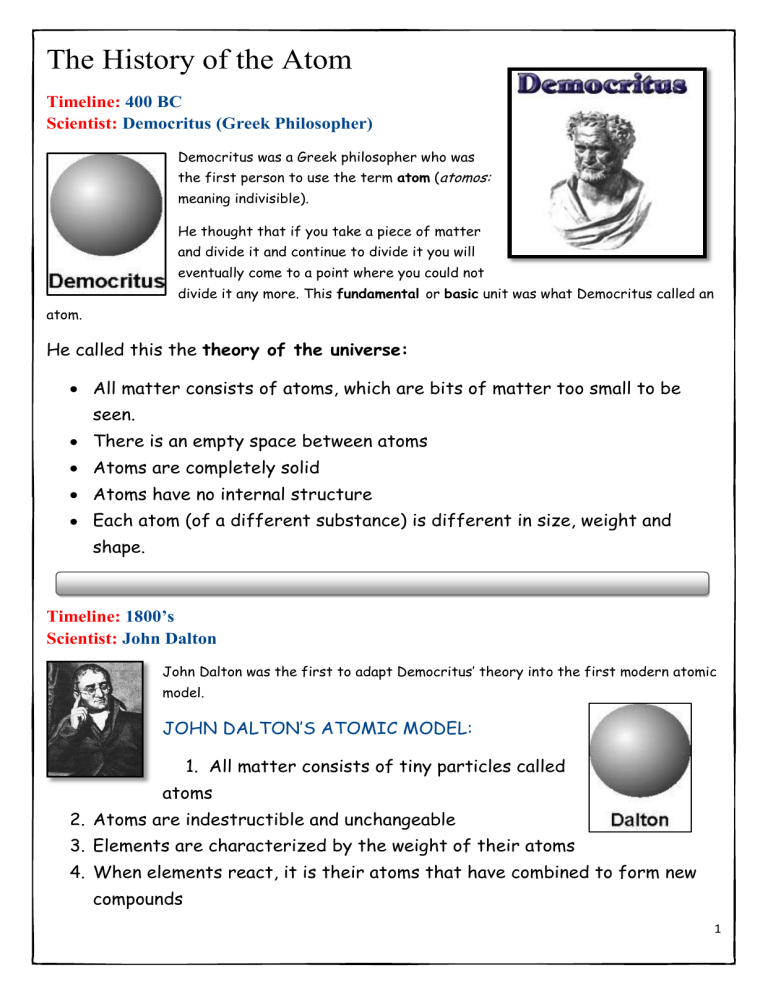
PDF The History of the Atom - Socorro Independent School District Each atom (of a different substance) is different in size, weight and shape. Timeline: 1800's Scientist: John Dalton John Dalton was the first to adapt Democritus' theory into the first modern atomic model. JOHN DALTON'S ATOMIC MODEL: 1. All matter consists of tiny particles called atoms 2. Atoms are indestructible and unchangeable 3.
What is the contribution of Democritus in atomic structure? What is the contribution of Democritus in atomic structure? Democritus was a central figure in the development of the atomic theory of the universe. He theorized that all material bodies are made up of indivisibly small " atoms .". Aristotle famously rejected atomism in On Generation and Corruption.
The Bohr model of the atom was proposed by Neil Bohr in 1915. It came into existence with the modification of Rutherford's model of an atom. Rutherford's model introduced the nuclear model of an atom, in which he explained that a nucleus which is positively charged is surrounded by negatively charged electrons.
PPT - Democritus PowerPoint presentation | free to view ... History of Atomic Theory - History of Atomic Theory Democritus 460-370 B.C Democritus said that matter was made of discrete particles he called atomos which means cannot be cut. | PowerPoint PPT presentation | free to view
7 Advantages and Disadvantages of Dalton's Atomic Theory Atom is a part of particle of a substance which is gas, liquid or solid (metal). The word of atom firstly punctuated by a philosopher named Democritus. Atom model is an illustration of atom based on theoretical studies that is supported by experiment.
compare & contrast - Atomic Theory Democritus and Aristotle greatest difference where their views with atoms.Democritus believed that the atom did exist and was the smallest unit of matter. Aristotle believe that there could be no the existence of the atom.Aristotle was incorrect , Democritus. they both developed their theory around 400 and 300 B.C in the era of ancient Greek philosophy
Democritus | Biography & Facts | Britannica Democritus, (born c. 460 bce—died c. 370), ancient Greek philosopher, a central figure in the development of philosophical atomism and of the atomic theory of the universe. Knowledge of Democritus's life is largely limited to untrustworthy tradition. It seems that he was a wealthy citizen of Abdera, in Thrace; that he traveled widely in the East; and that he lived to an advanced age.
Democritus - The Atomic Model Even though Democritus was the first to use the word atom he wasn't recognized for it and never had a atomic model or theory. Democritus' idea and use of the word "Atom" was the first step to building the foundation of chemistry with the atom thousands of years later!
Democritus Model | Explained Democritus, a Greek philosopher who lived in the fifth century BC, theorized about atoms and the specific materials they are made of.In Dalton's Model of the Atoms (ESAOA), he suggested that all matter consists of small things he called atoms. We know that atoms consist of a positively charged nucleus in the center, surrounded by negatively charged electrons.
Developing the atom - Models of the atom - AQA - GCSE ... The ancient Greek philosopher Demokritos (460-370 BCE) thought that matter was made up of millions of tiny, uncuttable pieces of that same matter. In fact, the word atom comes from the word...
The History Of The Atomic Model Timeline | Preceden Democritus's atomic theory stated that all matter is made up of small units called atoms which cannot be destroyed. Democritius' model is the earliest of the atomic model's written down. He was the first to use the term "atom."
› publication › 224831013(PDF) Introduction to Infrared Spectroscopy - ResearchGate The name atom was coined by Democritus [9] from the Greek, α-τ έ µ νω, meaning in Greek it cannot be cut any more or it is indivisible. This is the first time that it was postulated that
The Structure of an Atom Explained With a Labeled Diagram The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge.
Dalton's Model of the Atom and Early Atomic Theory Atoms are small, chemically indestructible particles of matter. Elements consist of atoms. Atoms of an element share common properties. Atoms of different elements have different properties and different atomic weights. Atoms that interact with each other obey the Law of Conservation of Mass. Essentially, this law states the number and kinds of atoms that react are equal to the number and kinds of atoms in the products of a chemical reaction.
democritus Flashcards and Study Sets | Quizlet J. J. Thomson. Robert Milikan (1909) First to propose that matter was made up of invisible particle…. All matter is made up of atoms (Solid Sphere Model) 1897 used cathode ray and positively charged plates to ( (disco…. Oil Drop Experiment; tiny oil droplets on metal plate with hol…. Democritus.


















0 Response to "37 democritus atom model diagram"
Post a Comment