36 lewis diagram for methane
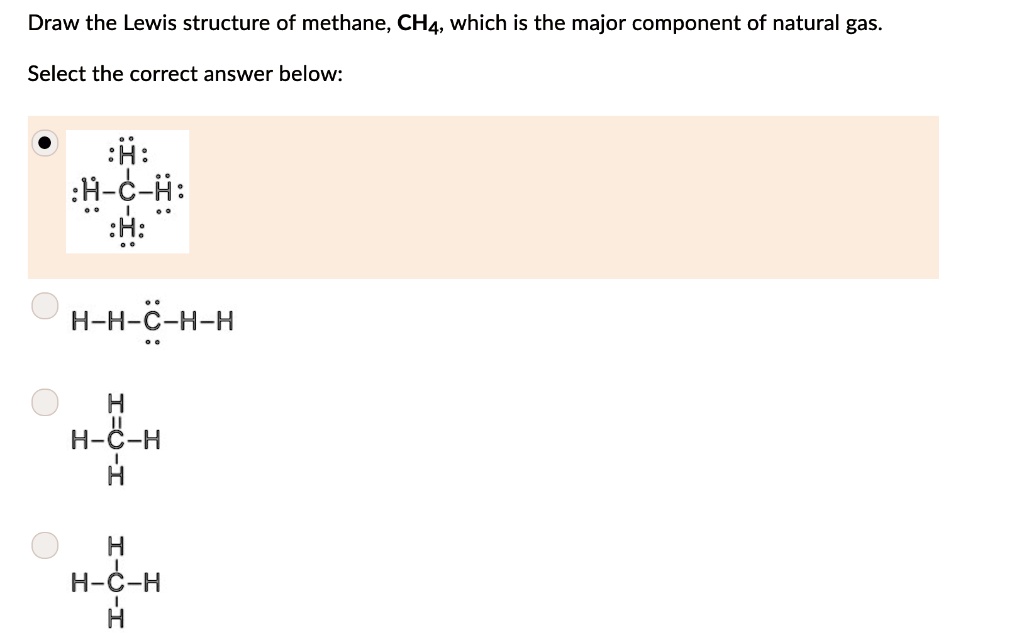
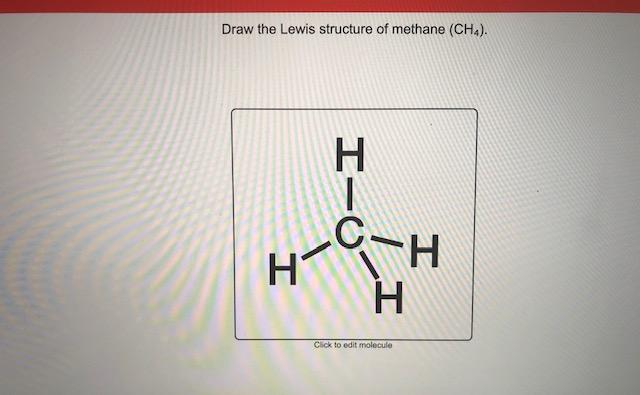
Answered: the Lewis structure of methane (CHA).… | bartleby the Lewis structure of methane (CHA). Click to draw a new structure. Question. Help me. Transcribed Image Text: the Lewis structure of methane (CHA). Click to draw a new structure Expert Solution. Want to see the full answer? Check out a sample Q&A here. See Solution. Want to see the full answer? Solved Draw the Lewis structure for methane (CHA) and ethane | Chegg.com Transcribed image text: Draw the Lewis structure for methane (CHA) and ethane (C2H6) in the box below. Then predict which would have the higher boiling point. Finally, explain how you came to that conclusion. CH4 C2H6 Same boiling point o Impossible to tell Explain here...
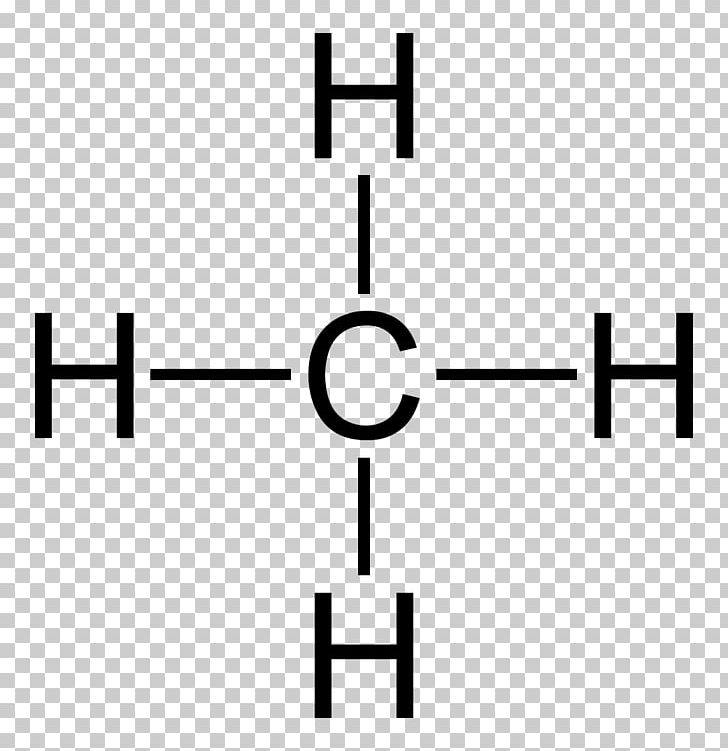
Lewis Structure for CH4 (Methane) - TerpConnect Drawing the Lewis structure for CH 4 (named methane) requires only single bonds. It's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. Video: Drawing the Lewis Structure for CH4
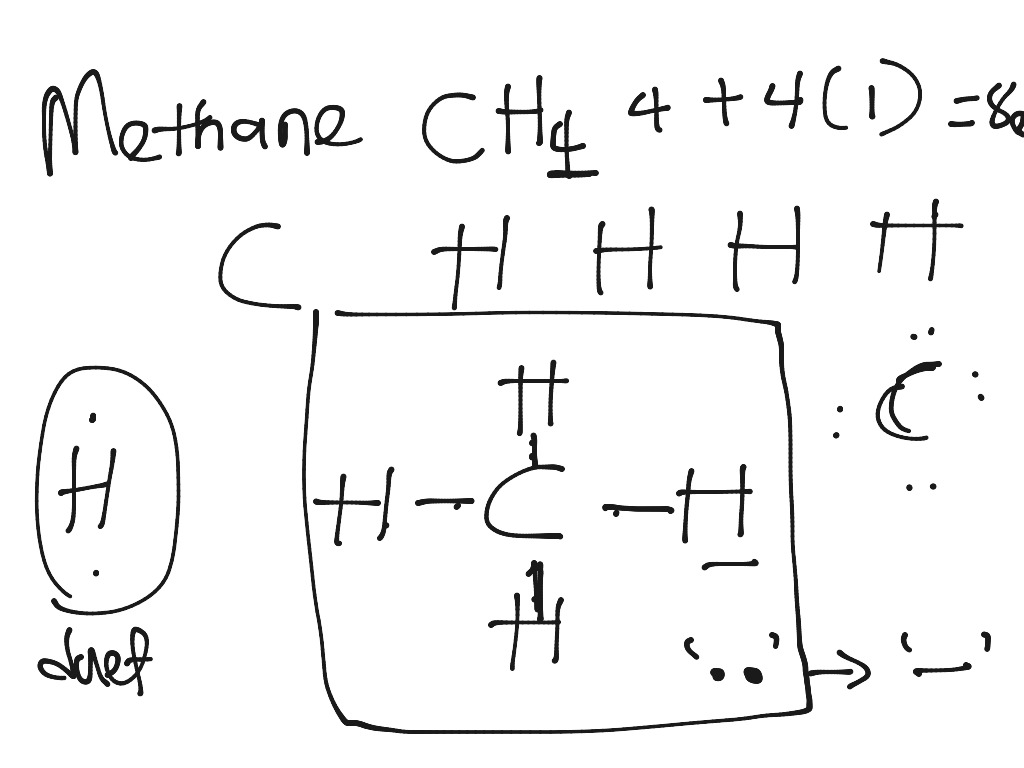
Lewis diagram for methane
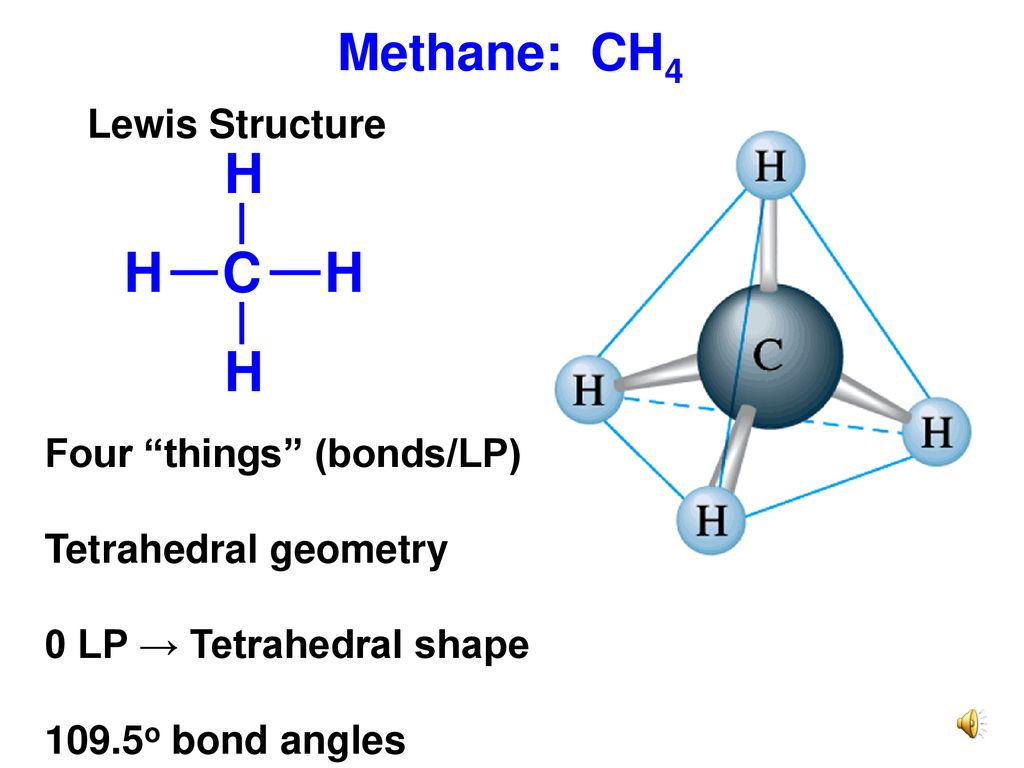
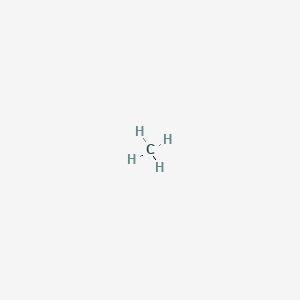
Draw Lewis dot diagram for the following. Methane (CH4) - Chemistry | Shaalaa.com Diagram. Draw Lewis dot diagram for the following. Methane (CH 4) Advertisement Remove all ads. CH4 Lewis Structure, Molecular Geometry, and Hybridization The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3. In a Lewis diagram for methane (CH4), which atom or atoms is inside, or central? A ... In the given molecule methane, CH₄: the valence configuration for C = 2s²2p² the valence configuration for H = 1s¹ Since two electrons are required to form a bond, the C atom in methane can form one bond with each of the 4 H atoms. Therefore, in the Lewis diagram, the C atom will be in the center surrounded by the 4 H atoms. Advertisement Survey
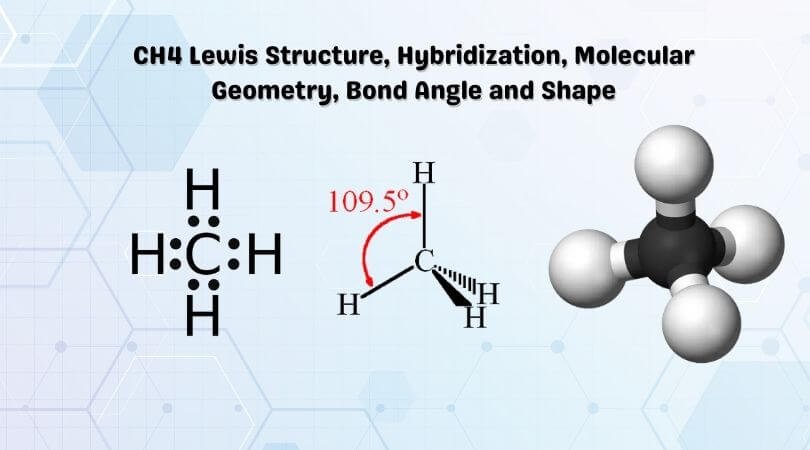
Lewis diagram for methane. Methane | CH4 - PubChem Methane | CH4 | CID 297 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards ... Lewis dot diagrams for the following:(a) Hydrogen (H2) (b) Water (H2O) (c) Carbon ... Click here👆to get an answer to your question ️ Lewis dot diagrams for the following:(a) Hydrogen (H2) (b) Water (H2O) (c) Carbon dioxide (CO2) (d) Methane (CH4) CH4 Lewis Structure, Hybridization, Molecular Geometry, Bond Angle and Shape So in the Lewis structure of CH4 or Methane, there are four single or covalent bonds between each Hydrogen and Carbon atom. There are four bonding pairs of electrons and no lone pair of electrons in this molecule. CH4 Hybridization The Lewis Dot Structure for CH4 - MakeTheBrainHappy The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots. Each atom in the bond has a full valence, with carbon having access to eight electrons and each hydrogen having access to two (this is why hydrogen only needs two).The covalent bonds between the C and the H are similar to the ones formed between two Hs ...
Draw Lewis Structure For Ch4 - Nelson Tardwilis The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3. Electron Dot Diagram For Methane - schematron.org Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) . Lewis dot dragram for methane: Methane, with molecular formula CH4, is shown. It is important to remember that Lewis valence dot diagrams are models that Methane is the main component of natural gas, and its chemical formula is CH4. What is the Lewis Structure for Methane? - Answers Hi, I am having trouble with chemistry an I need to know What the Lewis Structure for CH4 or Methane is an the number of groups on the central atom and the Number of bonding groups on the central ... Draw the Lewis structure for methane (CH4) and ethane (C2H6) in the box below. Then ... The Lewis structure shows the valence electrons in a molecule.Ethane will have a higher boiling point than methane.. We can deduce the number of valence electrons in a molecule by drawing the Lewis structure of the molecule. The Lewis structure consists of the symbols of elements in the compound and the valence electrons in the compound.
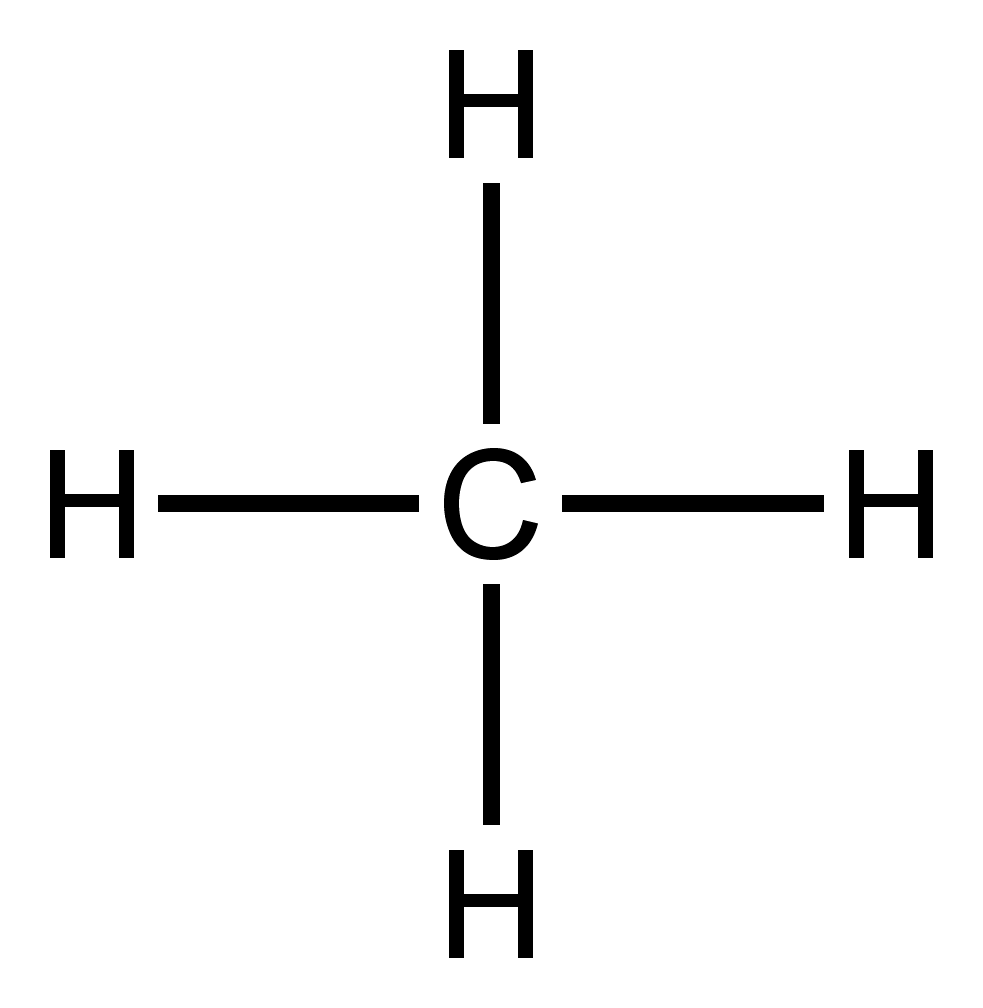
Methane (CH4) Molecule Lewis Structure CH 4 lewis structure There are following specifications in the lewis structure of methane. Four hydrogen atoms have made bonds with center carbon atom and all those bonds are single bonds. No lone pairs on valence shells of carbon and hydrogen atoms. Oxidation number of carbon atom is -4. Steps of drawing lewis structure of CH 4 molecule Answered: Draw the Lewis structure of methane… | bartleby Science Chemistry Q&A Library Draw the Lewis structure of methane (CH4). Draw the Lewis structure of methane (CH4). Question. thumb_up 100%. Help me. Transcribed Image Text: Draw the Lewis structure of methane (CH4). Expert Solution. Want to see the full answer? Check out a sample Q&A here. Lewis Dot Structures 1. Methane 4 - Houston Community College Methane 4 Lewis Dot Structures 1. Methane - CH 4 Number of Valence Electrons: 4 from C and 1 each from 4 H = 8 Carbon is more electronegative than hydrogen, but hydrogen can never be the "central" atom, as it can only form 1 bond. Carbon always forms 4 bonds (2 electrons each). 2. Ammonia - NH 3 How to draw methane ch4 lewis structure - SlideShare Steps to write the Lewis structure of methane 1. Find the number of valence electrons for each of the atoms in the molecule. The number of valence electrons is usually the same as the group number where the element is located. 3. The number of valence electrons for hydrogen is 1 (group 1) 4. The number of valence electrons for carbon is 4 ...
Lewis dot structure of methane - fornoob.com Lewis dot structure of methane. December 27, 2021 thanh. below is the Lewis structure of chlorine. Below is the Lewis structure of the methane (CH_4) molecule Count the number of bonding pairs and the number of lone pairs around the carbon atom.
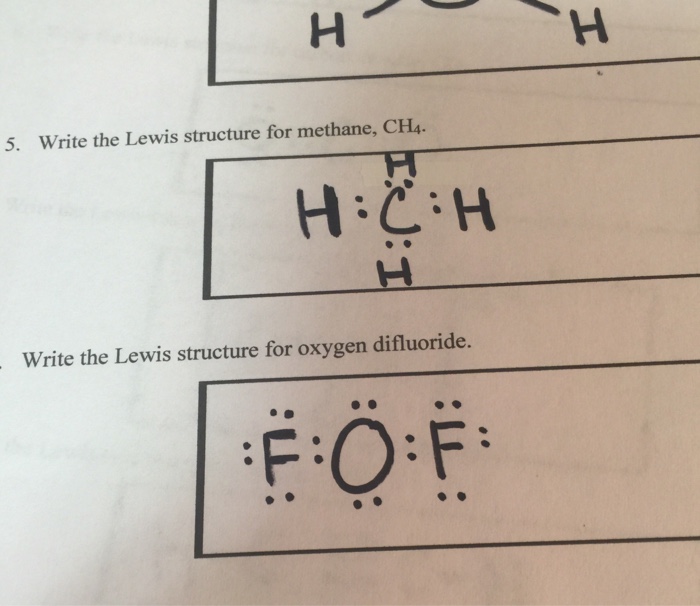
What is the Lewis structure of methane? | Study.com The Lewis dot structure for methane (CH 4) is: This is derived by following 5 general steps for writing Lewis dot structures. First, we need to... See full answer below.
Lewis Dot Structure of CH4 (methane) - YouTube I quickly take you through how to draw the Lewis Structure of methane, CH4. I also go over hybridization, shape and bond angle.
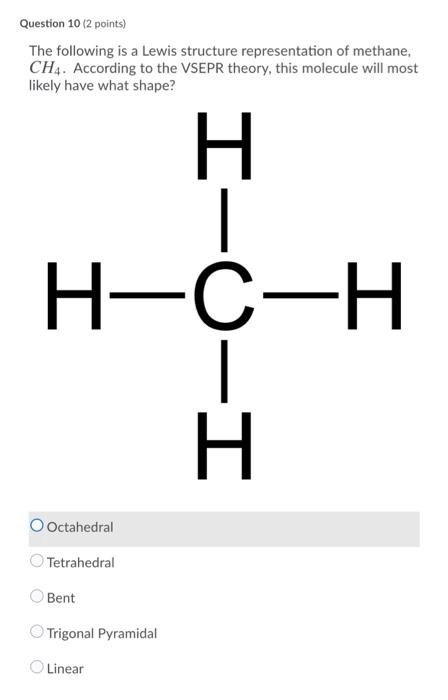
OneClass: Draw the lewis dot structure for methane, . According to VSEPR theory, what ... Draw the lewis dot structure for methane, . According to VSEPR theory, what is the molecular geometry of methane? What are the predicted bond angles? Answer +20. Watch. 1. answer. 0. watching. 235. views. For unlimited access to Homework Help, a Homework+ subscription is required.
Lewis Dot Diagram Ch4 Drawing the Lewis structure for CH 4 (named methane) requires only single diagramweb.net's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. Lewis dot structure of CH 4.
CH4 Lewis Structure - How to Draw the Dot Structure for CH4 (Methane) - YouTube How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use...
Methane (CH4) lewis dot structure, molecular geometry, electron geometry, polar or ... How to draw lewis structure of CH4 (Methane)? CH4 lewis's structure is very simple and easy to draw, it is made up of one carbon atom that takes the central position and four hydrogens that spread around the central atom. There are no lone pair electrons present in the lewis dot structure of CH4.
Ch4 Electron Dot Diagram Drawing the Lewis structure for CH 4 (named methane) requires only single schematron.org's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. The Lewis Dot Structure for CH4 is shown above.
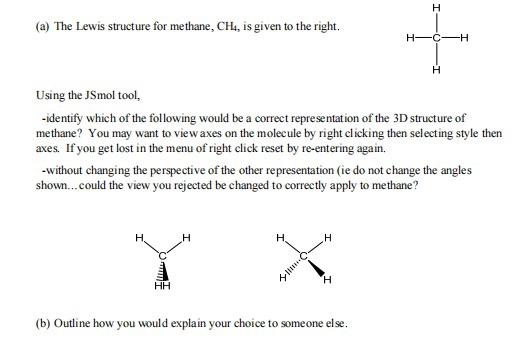
Solved H (a) The Lewis structure for methane, CH4, is given | Chegg.com Chemistry questions and answers. H (a) The Lewis structure for methane, CH4, is given to the right. H CH Using the JSmol tool, -identify which of the following would be a correct representation of the 3D structure of methane? You may want to view axes on the molecule by right clicking then selecting style then axes.
In a Lewis diagram for methane (CH4), which atom or atoms is inside, or central? A ... In the given molecule methane, CH₄: the valence configuration for C = 2s²2p² the valence configuration for H = 1s¹ Since two electrons are required to form a bond, the C atom in methane can form one bond with each of the 4 H atoms. Therefore, in the Lewis diagram, the C atom will be in the center surrounded by the 4 H atoms. Advertisement Survey
CH4 Lewis Structure, Molecular Geometry, and Hybridization The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3.
Draw Lewis dot diagram for the following. Methane (CH4) - Chemistry | Shaalaa.com Diagram. Draw Lewis dot diagram for the following. Methane (CH 4) Advertisement Remove all ads.


















0 Response to "36 lewis diagram for methane"
Post a Comment