wou.edu › chemistry › coursesCH150: Chapter 2 – Atoms and Periodic Table – Chemistry How many electrons are in the valence shell of Phosphorus? Phosphorus has an electron configuration of: P = 1s 2 2s 2 2p 6 3s 2 3p 3 Solution. In the analysis of the electron configuration of phosphorus, we can see that the 3rd shell is the valence shell. For phosphorus there are 2 electrons in the 3s orbital, and there are 3 electrons in the ... en.wikipedia.org › wiki › Electron_configurationElectron configuration - Wikipedia Physicists and chemists use a standard notation to indicate the electron configurations of atoms and molecules. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. for phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript.
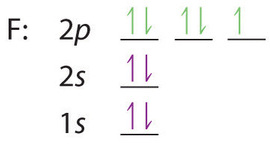
Use the orbital-filling diagram to show the electron configuration of phosphorus, p.
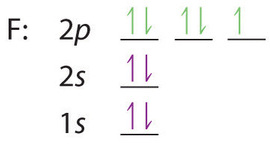
6.8: Electron Configurations - Chemistry LibreTexts

Electron Configuration Activity & Worksheets | Teachers Pay ...

CHEM 1A: GENERAL CHEMISTRY Chapter 8: Electron Configuration ...

6.4 Electronic Structure of Atoms (Electron Configurations ...

Electron Configuration for Oxygen (O)

13 Electron Configuration-S

Difference Between Orbital Diagram and Electron Configuration ...

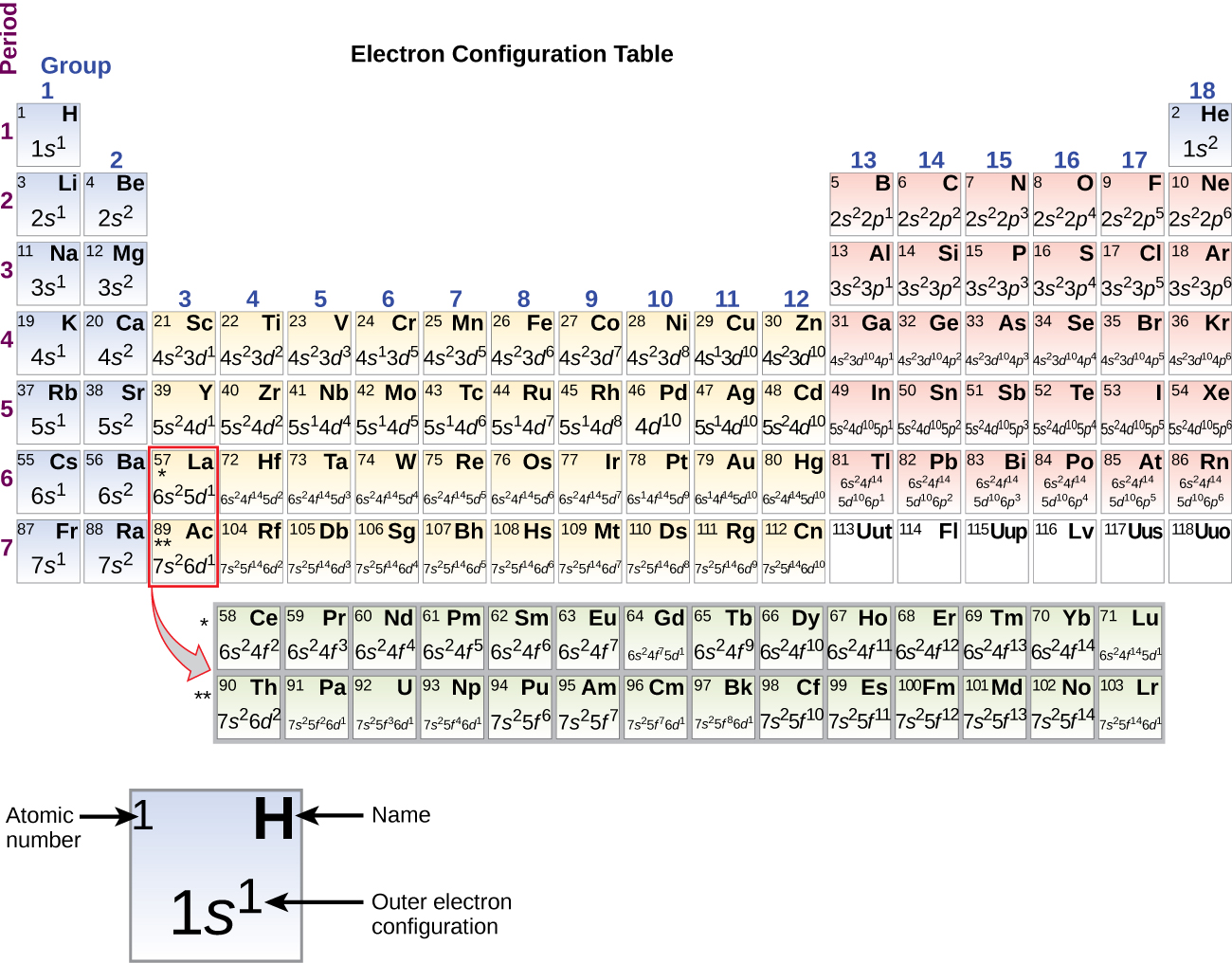
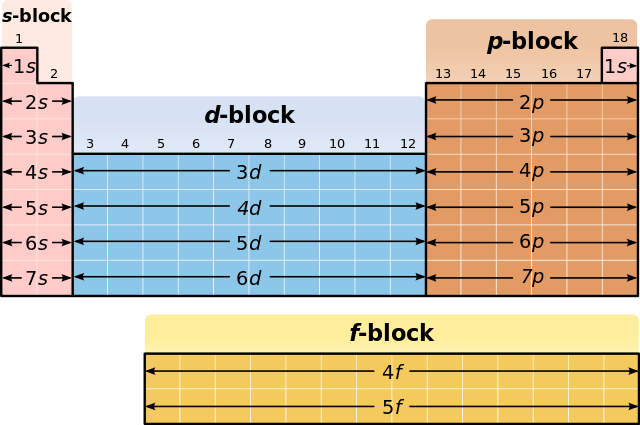
Electron Configurations & The Periodic Table

Chem Ch 3, Chem Ch 2 Flashcards | Quizlet
6.4 Electronic Structure of Atoms (Electron Configurations ...

Worksheet 4-1
CH104 – Chapter 2: Atoms and The Periodic Table – Chemistry

Orbital Diagrams — Overview & Examples - Expii

Orbital Diagrams and Electron Configuration - Basic Introduction - Chemistry Practice Problems

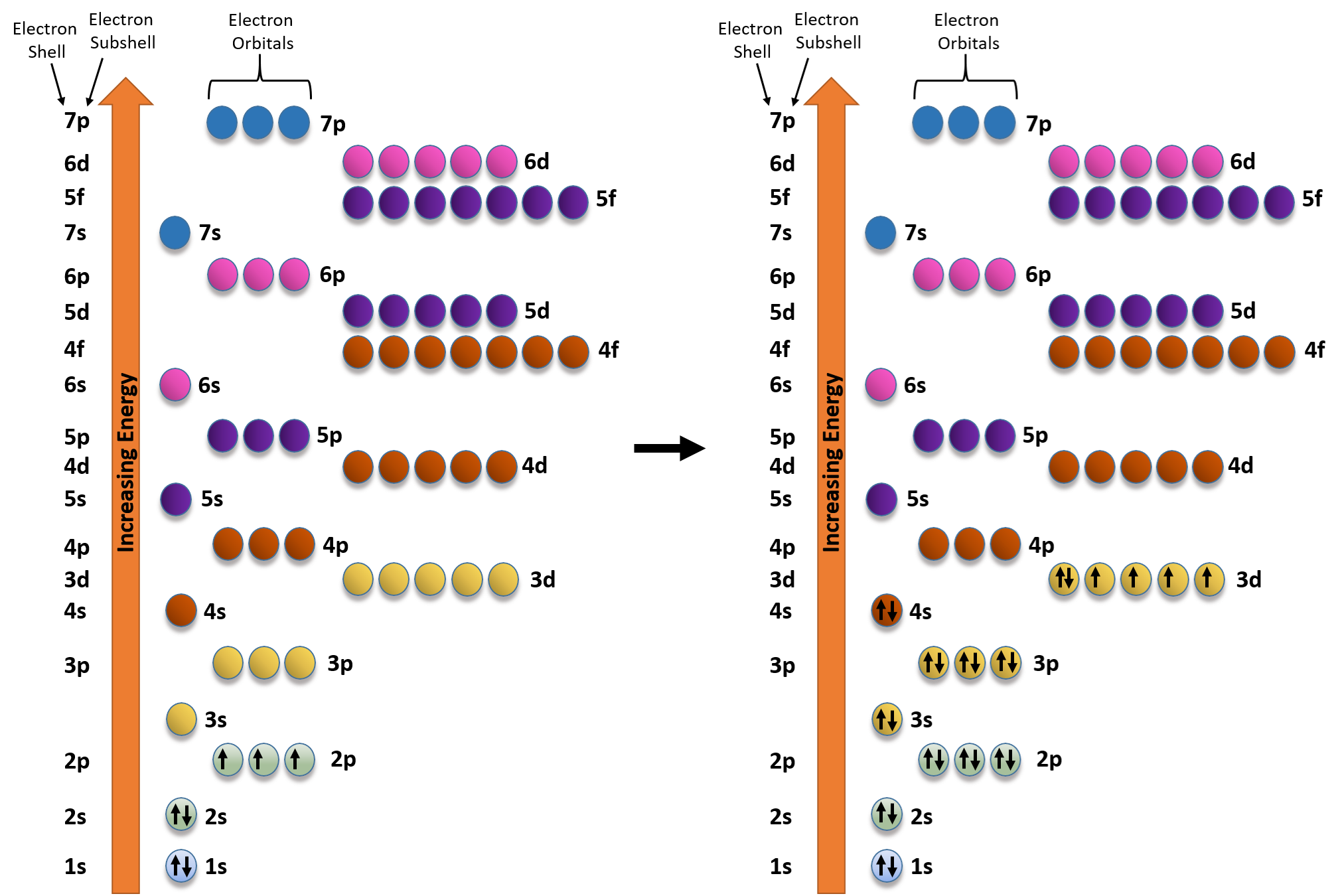
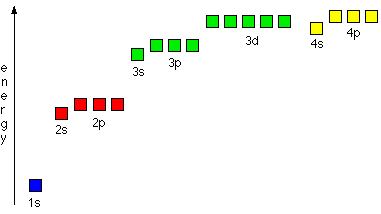
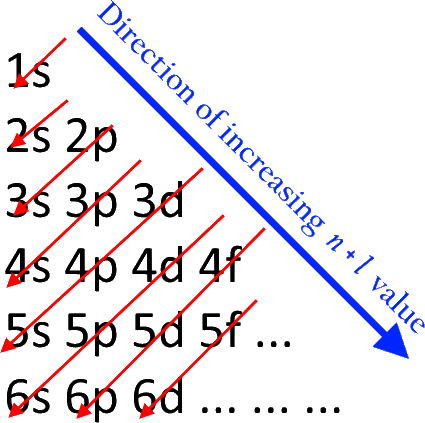
The Building-Up (Aufbau) Principle | Introduction to Chemistry

Electron configuration - Wikipedia

Review I Constants I Show the orbital-filling diagram for S ...

Chapter Two Atoms and the Periodic Table Fundamentals of ...
How to Write an Electron Configuration | ChemTalk
Question #37699 | Socratic
How do you determine the electron configuration of Fe? | Socratic

Electron configurations for the second period
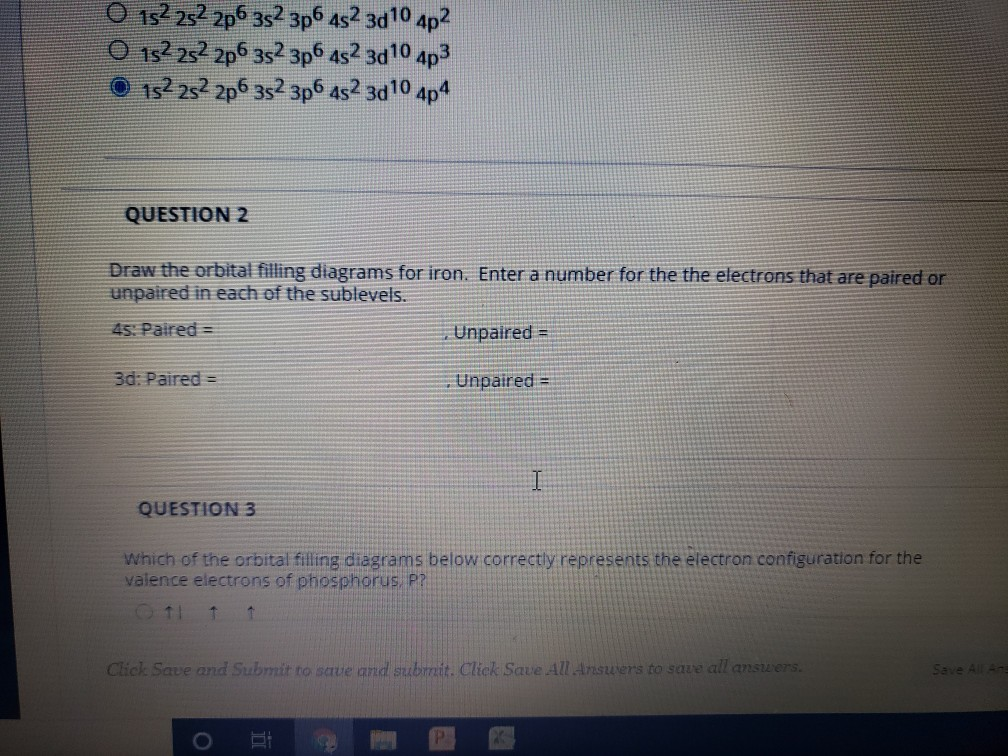
Solved O 152252 206 352 3p6 452 3d 10 4p2 0 152 252 2p6 352 ...

Untitled

Orbital Diagrams — Overview & Examples - Expii

Electron Configurations, how to write out the s p d f ...

Hund's Rule and Orbital Filling Diagrams | Chemistry for Non ...

Orbital Diagrams — Overview & Examples - Expii
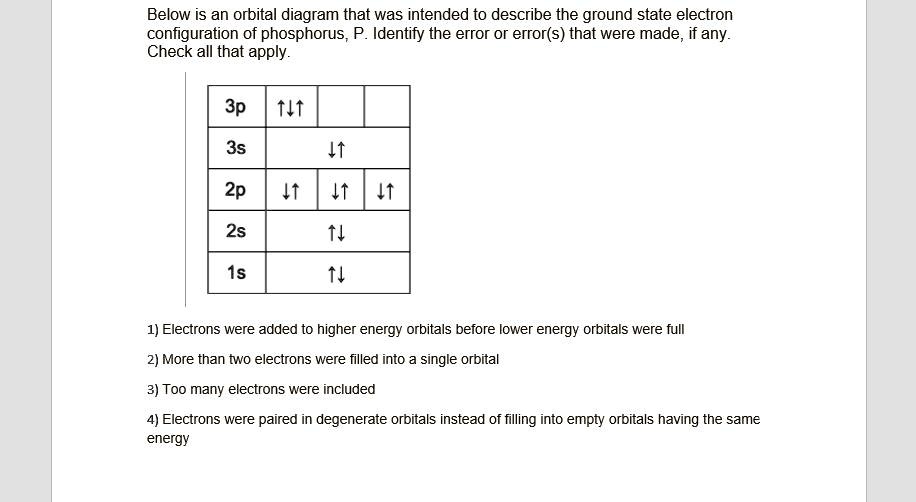
SOLVED:Below is an orbital diagram that was intended to ...

Dublin Schools - Lesson : Orbital diagrams and Electron ...

Electron configuration - Wikipedia

Build the orbital diagram for the ion most likely formed by ...

Chem Ch 3, Chem Ch 2 Flashcards | Quizlet

SOLVED:Seledt the singke best answer Choose the correct ...
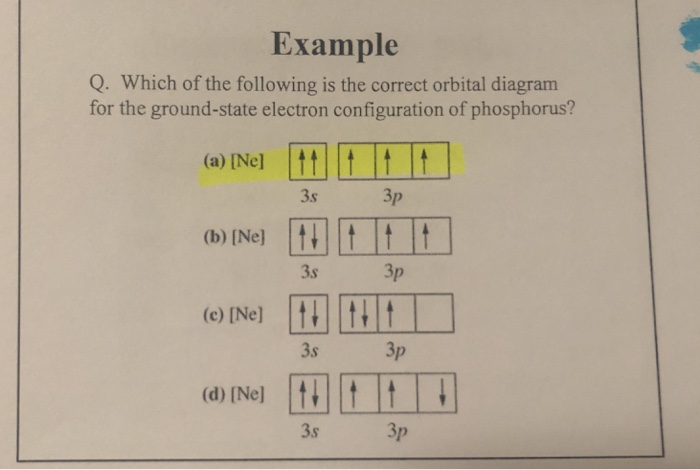
Solved Example Q. Which of the following is the correct ...

6.4 Electronic Structure of Atoms (Electron Configurations ...
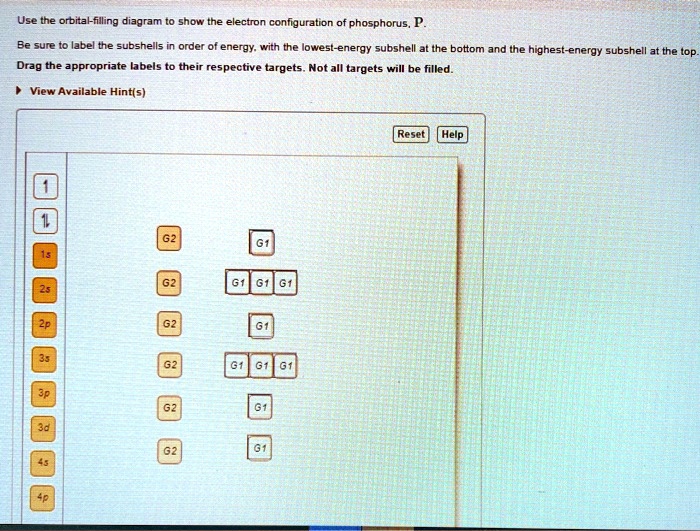
SOLVED:Use the orbralfilling diagram show the electron conf g ...

4 Ways to Write Electron Configurations for Atoms of Any Element

Test 3: Practice-2

MakeTheBrainHappy: How many valence electrons are in an atom ...
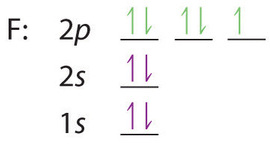
































0 Response to "40 use the orbital-filling diagram to show the electron configuration of phosphorus, p."
Post a Comment