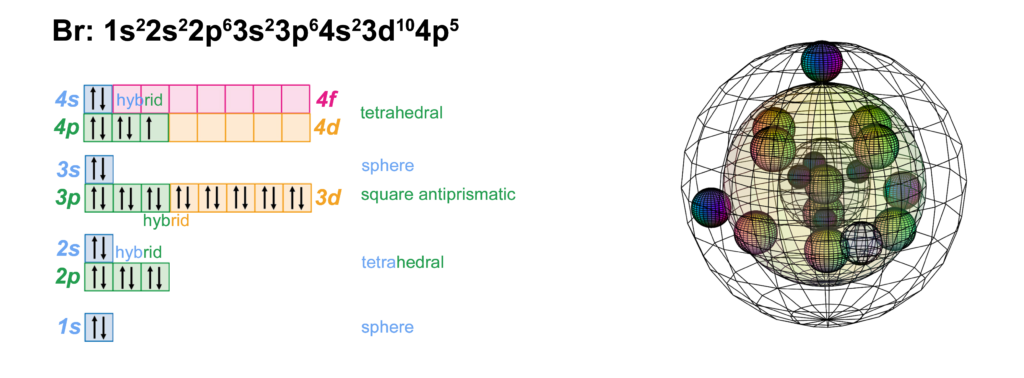
39 show the orbital filling diagram for br bromine
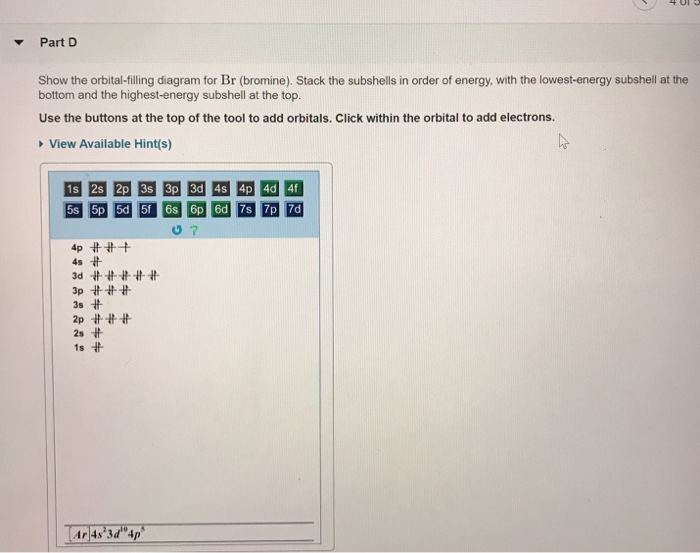
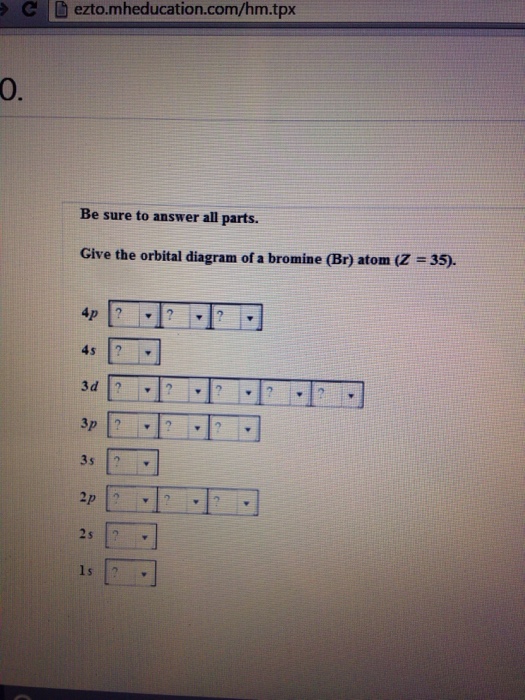
Solved Part D Show the orbital-filling diagram for Br ... Question: Part D Show the orbital-filling diagram for Br (bromine). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint (s) Reset Help 1 18 25 2p 3s 3p 30 48 4p Submit Provide Feedback Next > Part B Show the orbital-filling diagram for N (nitrogen). Show The Orbital-filling Diagram For Br (bromine) Orbital-Filling Diagram for Bromine. Bromine has 35 electrons, so it will have 35 arrows placed in its orbital-filling diagram as in figure The order bottom to top . Explanation: All you need to do is work your way across the periodic table filling the orbitals as you go. The full version of this is.
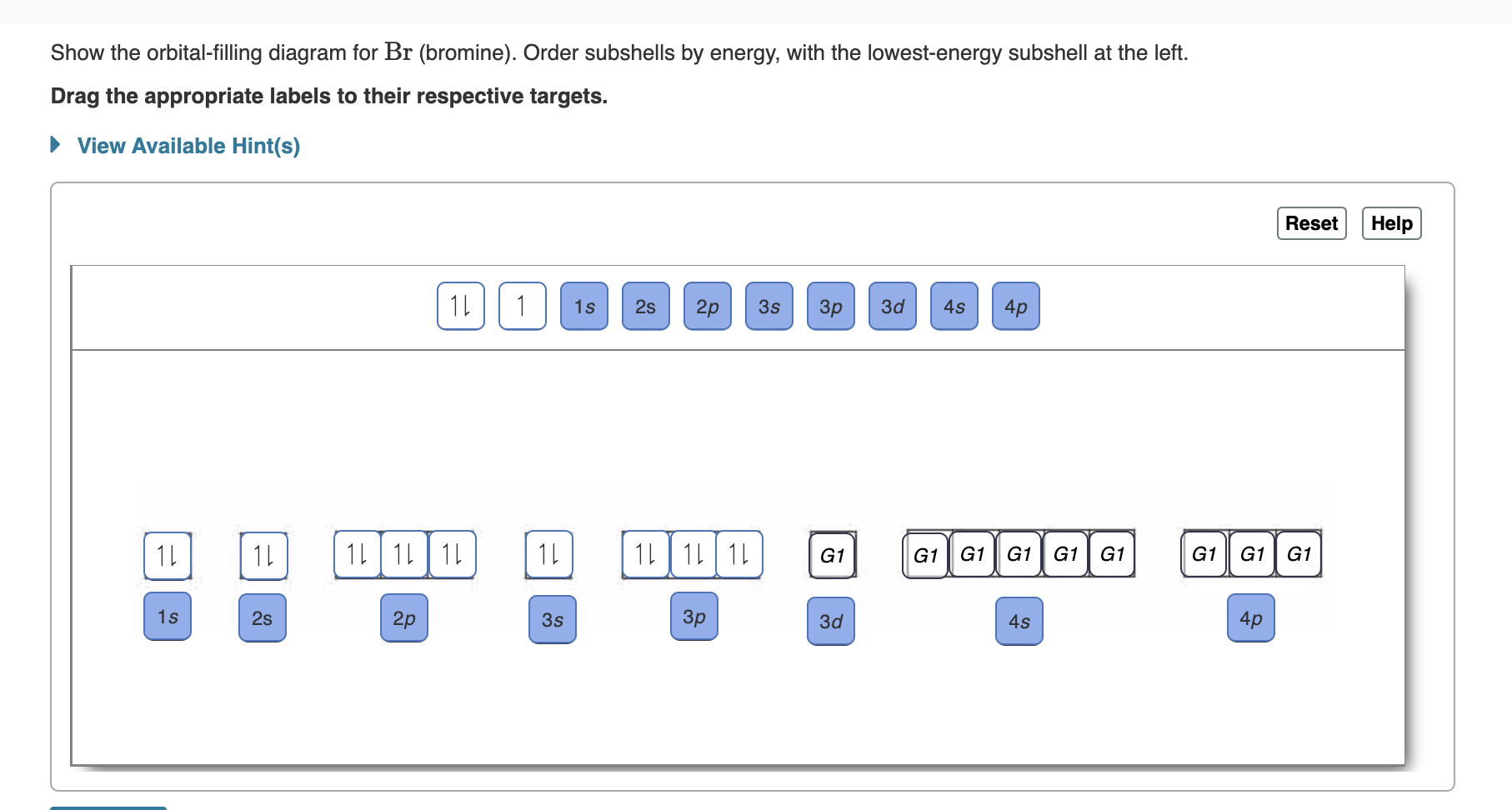
Orbital Filling Diagram For Bromine Orbital-Filling Diagram for Bromine. Bromine has 35 electrons, so it will have 35 arrows placed in its orbital-filling diagram as in figure The order bottom to top .Show the orbital-filling diagram for Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top.
Show the orbital filling diagram for br bromine
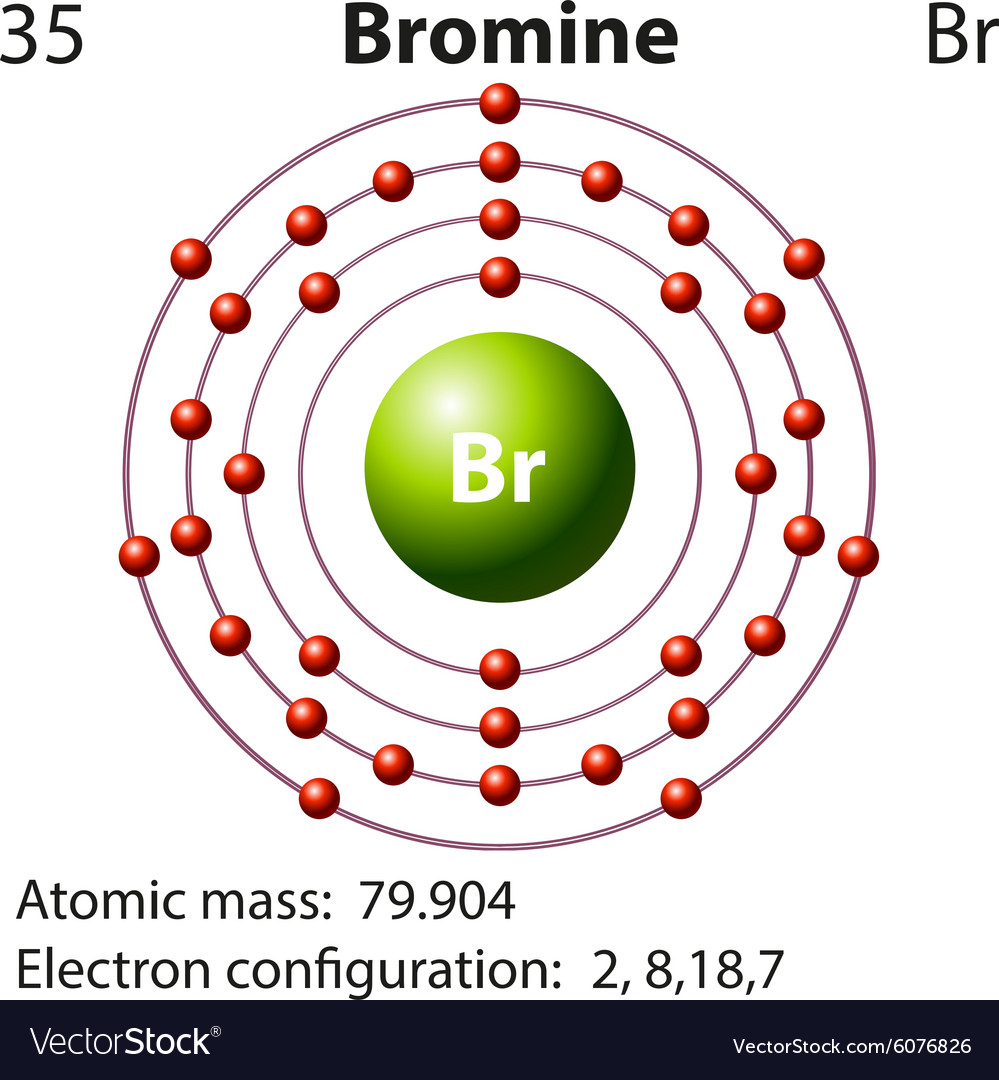
Show The Orbital-filling Diagram For Br (bromine) The orbital diagram for bromine shows four concentric circles around a dot representing a nucleus, with two dots on the first circle, eight on the second, 18 on the third and seven on the fourth. Each dot on a circle represents an electron. The four concentric circles represent the four energy levels in which the electrons of a bromine atom sit. Chem Unit 6 Flashcards - Quizlet Show the orbital-filling diagram for Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Relating Quantum Numbers and Electron Configurations to the Periodic Table Show The Orbital Filling Diagram For Br Bromine What Is the Orbital Diagram for Bromine? The orbital diagram for bromine shows four concentric circles around a dot representing a nucleus, with two dots on the first circle, eight on the second, 18 on the third and seven on the fourth. Each dot on a circle represents an electron.
Show the orbital filling diagram for br bromine. Show The Orbital Filling Diagram For Br Bromine stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy.the orbital doagram for bromine is the orbital diagram for bromine is the orbital diagram for bromine is mar 22, · best answer: yes; bromine (atomic number 35) has 1 less electron than the next higher inert gas, krypton (atomic number 36) … Answered: Show the orbital-filling diagram for Br… | bartleby Show the orbital-filling diagram for Br (bromine). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. • View Available Hint(s) Reset Help 11| 1 1s 2s 2p 3s Зр 3d 4s 4p 1L 1L | 1| 11 11 | 11 | 1 G1 G1 G1 G1 G1 G1 G1 G1 G1 1s 2s 2p 3s 3p 3d 4s 4p 11 What is the orbital diagram for bromine? - Answers The orbital doagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-7. Study Chem Ch 2 Flashcards | Quizlet Show the orbital-filling diagram for Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Identify the specific element that corresponds to the following electron configuration:
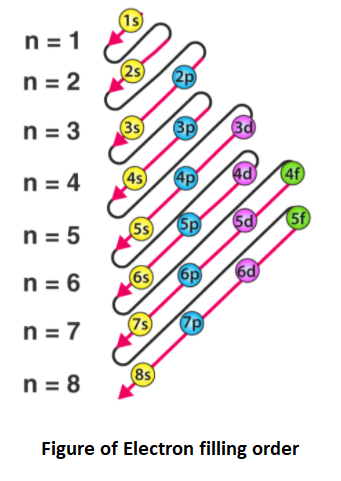
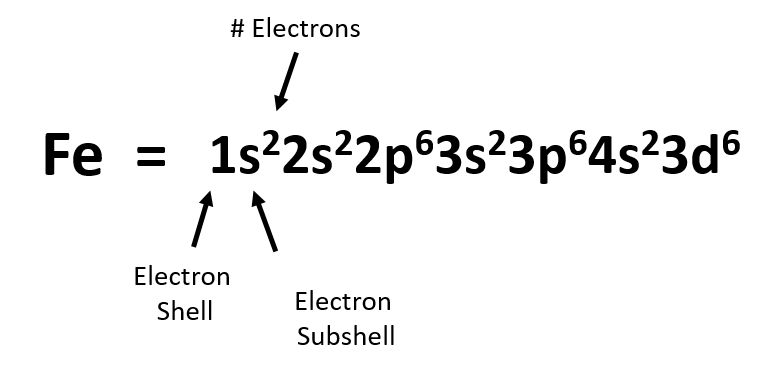
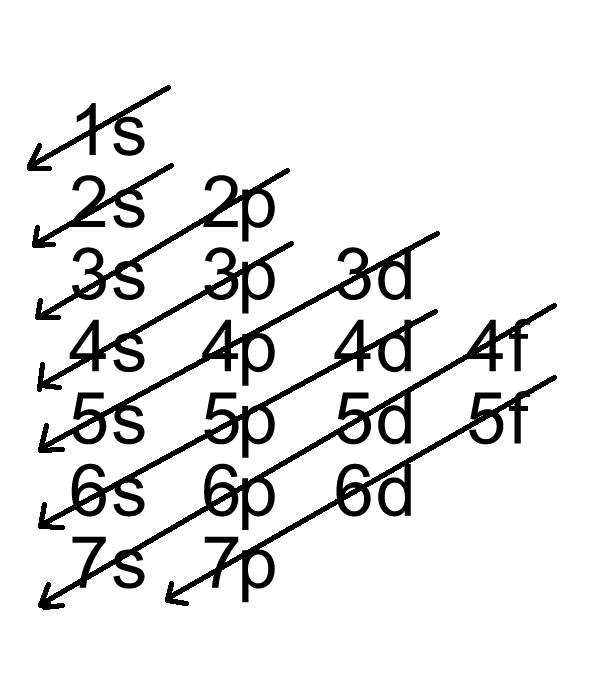
Solved Part D Show the orbital-filling diagram for Br ... Part D Show the orbital-filling diagram for Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons View Available Hint (s) 困囚 4s 3p ### 3s # 2p### 2s # 1s Show the orbital-filling diagram for Br (b... | Clutch Prep Show the orbital-filling diagram for Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Learn this topic by watching The Electron Configuration Concept Videos. Orbital Diagram For Bromine, Atomic Numbers Electron ... The adsorption mechanism indicated that PtIV was reduced to PtII and then coordinated with N. This text is conformed to ideal syllabi of B.Sc. Show the orbital-filling diagram for Br bromine. I wouldn't expect there to be any non-bonding orbitals populated either, as this is a simple diatomic we're dealing with and the atoms involved are identical. Bromine(Br) electron configuration and orbital diagram The 3d orbital is now full. So, the remaining five electrons enter the 4p orbital. Therefore, the bromine(Br) electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. How to write the orbital diagram for bromine(Br)? To create an orbital diagram of an atom, you first need to know Hund's principle and Pauli's exclusion principle.
Part D. Show the orbital-filling diagram f... | Clutch Prep Part D. Show the orbital-filling diagram for Br (bromine). Order subshells by energy, with the lowest-energy subshell at the left. To learn to create orbital-filling diagrams. An orbital-filling diagram shows the number of electrons in each orbital, which are shown in order of energy. Show The Orbital Filling Diagram For Br Bromine What Is the Orbital Diagram for Bromine? The orbital diagram for bromine shows four concentric circles around a dot representing a nucleus, with two dots on the first circle, eight on the second, 18 on the third and seven on the fourth. Each dot on a circle represents an electron. Chem Unit 6 Flashcards - Quizlet Show the orbital-filling diagram for Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Relating Quantum Numbers and Electron Configurations to the Periodic Table Show The Orbital-filling Diagram For Br (bromine) The orbital diagram for bromine shows four concentric circles around a dot representing a nucleus, with two dots on the first circle, eight on the second, 18 on the third and seven on the fourth. Each dot on a circle represents an electron. The four concentric circles represent the four energy levels in which the electrons of a bromine atom sit.

























0 Response to "39 show the orbital filling diagram for br bromine"
Post a Comment