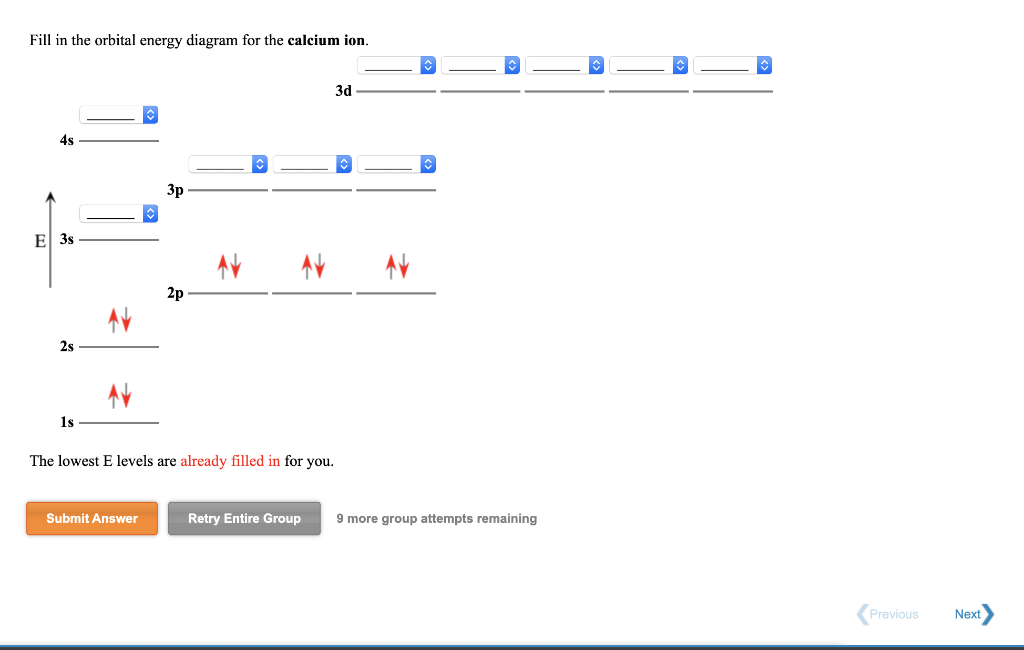
36 orbital filling diagram for calcium
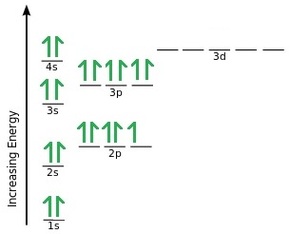
the order of filling 3d and 4s orbitals - chemguide The diagram (not to scale) summarises the energies of the orbitals up to the 4p level. The oddity is the position of the 3d orbitals. They are shown at a slightly higher level than the 4s - and so it is the 4s orbital which will fill first, followed by all the 3d orbitals and then the 4p orbitals. 5.17 Hund's Rule and Orbital Filling Diagrams Flashcards ... How many unpaired electrons does calcium have? You may need to draw the orbital filling diagram for the last sublevel that contains electrons. 0. The number of unpaired electrons in an atom can be determined from the orbital filling diagrams and correctly applying Hund's rule. Boron has one unpaired electron, while carbon has two, and nitrogen ...
Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...
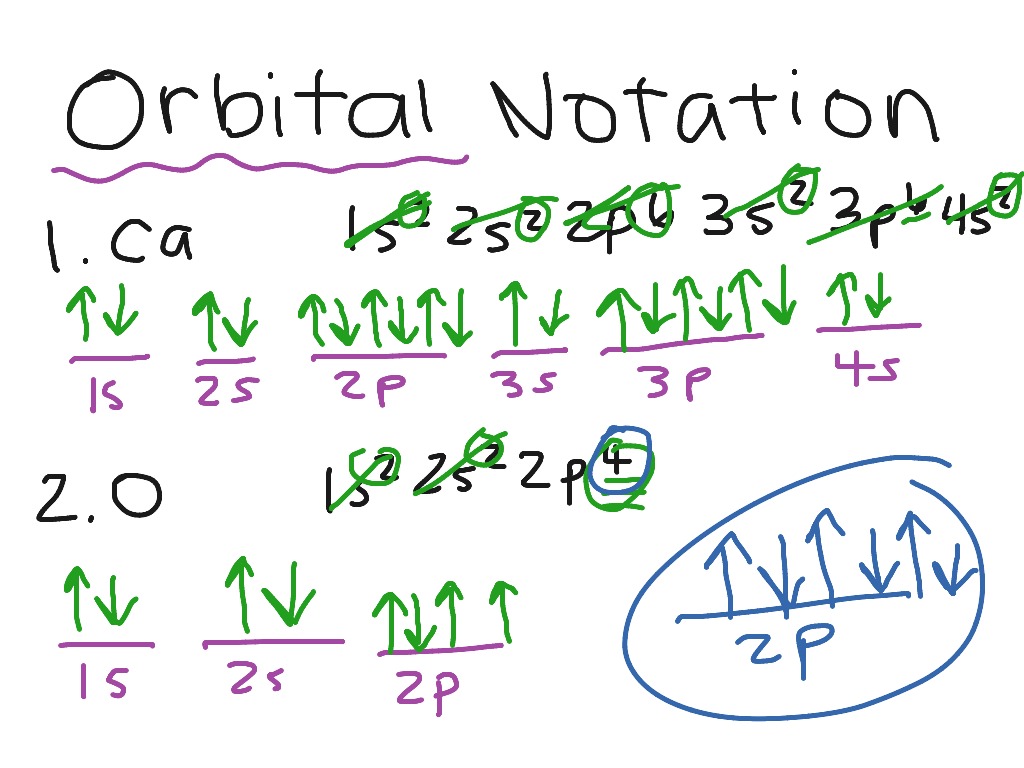
Orbital filling diagram for calcium
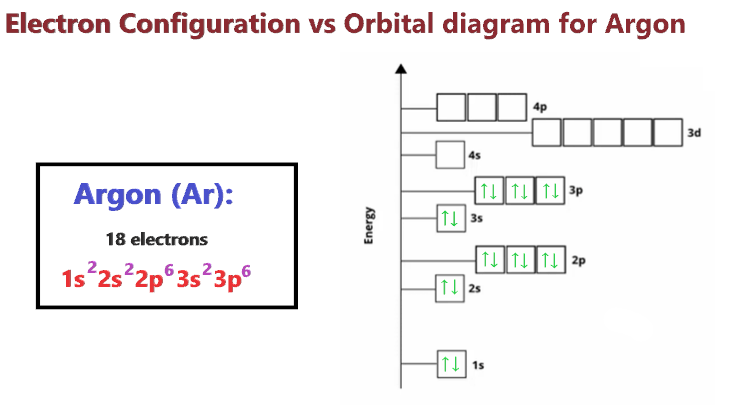
Sodium Orbital diagram, Electron configuration, and ... The orbitals are 1s, 2s, 2p, and 3s. The Sodium orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, the six electrons in 2p orbital, and the remaining one electron in 3s orbital. Orbital diagram for a ground-state electron configuration of Sodium atom is shown below-. PDF Orbital Filling Diagrams - Oak Park USD Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom PDF Electron Configurations and Orbital Diagrams key 1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals?
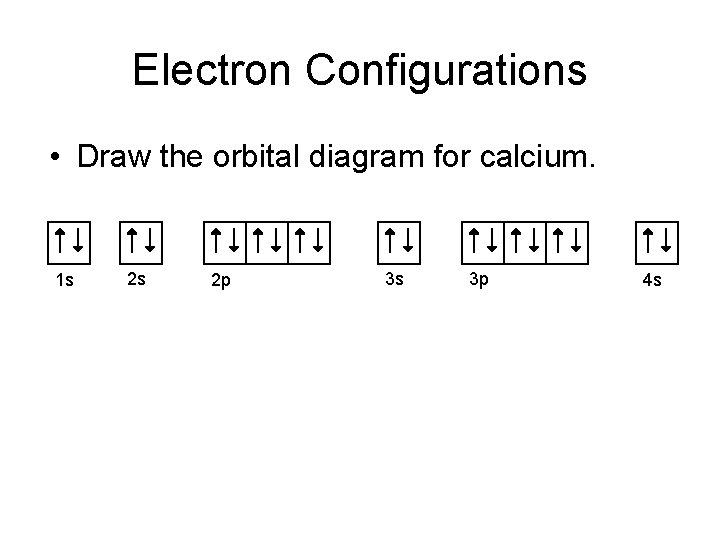
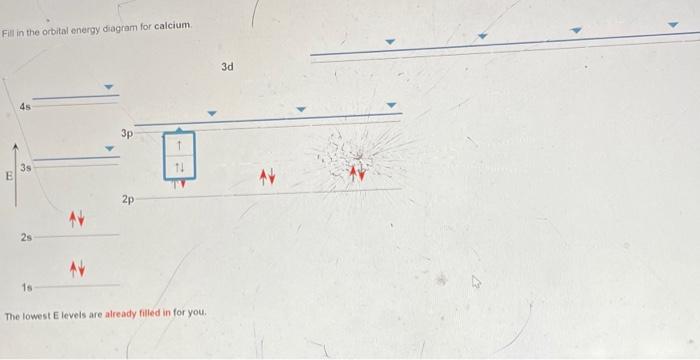
Orbital filling diagram for calcium. Calcium Orbital Filling Diagram Oct 31, 2018 · Ca electron occupancy The orbital notation of calcium (Ca) is 1s2 2s2 2p6 3s2 3p6 4s2 Or short-hand: [Ar] 4s2. The diagram (not to scale) summarises the energies of the orbitals up to the 4p level. The oddity is the position of the 3d orbitals. PDF Electron Config & Orbital Filling Answer Key 1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following ... Electron Configuration for Calcium (Ca) In writing the electron configuration for Calcium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Calcium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. Orbital Filling Diagram For Calcium Jan 14, 2019 · Orbital diagrams are pictorial representations of the arrangement of Rather than filling the orbitals one-by-one, it is easier to note that calcium is in the s- block. When we write the configuration we'll put all 20 electrons in orbitals around the nucleus of the Calcium atom. Here I use the electron.
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12 ... PDF Electron Configuration Practice Worksheet - Weebly 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium Atomic Orbital Diagram of Calcium (2+) Ion - YouTube Is this the correct atomic orbital diagram for a calcium (2+) ion? Please indicate "true" or "false" accordingly on your Google quiz form. The Order of Filling 3d and 4s Orbitals | ChemKey The Order of Filling Orbitals. Electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. Where there is a choice between orbitals of equal energy, they fill the orbitals singly as far as possible. The diagram (not to scale) summarises the energies of the orbitals up to the 4p level.
How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. What is the electron configuration of CA? - MSI Which is the correct orbital configuration for calcium? Ca electron occupancy The orbital notation of calcium (Ca) is 1s2 2s2 2p6 3s2 3p6 4s2 Or short-hand: [Ar] 4s2. The orbital filling diagram of lithium. The electron configuration of lithium is 1s²2s¹. How many electrons are in a degenerate orbital? The second electron will be of opposite ... PDF Work on Elctron configuration and orbital diagram 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium PDF Orbital Diagram For Ca2 - graylog.dsc.net community college, orbital filling diagrams for ca amp ca2 yahoo answers, solved a write the electron configuration for co2 expr, what is the electron configuration for a nitride ion, electron configuration and orbital filling calcium ca amp chlorine cl, chemistry chapter 4 and 5 flashcards
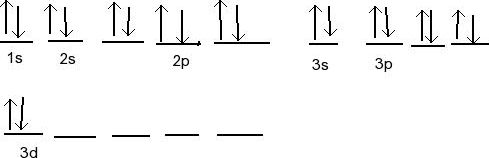
orbital-diagram.pdf - 2s 1s 2s 1s 2s 1s 2p 2p 2p 2p 2p 2p ... Printable Worksheets @ Hund's Rule & Orbital Filling Diagram Complete the orbital diagram for each element. 2) calcium 1s 2s 4s 3s 3d 2p 4p 3p 1) sodium 1s 2s 4s 3s 3d 2p 4p 3p 3) nickel 1s 2s 4s 3s 3d 2p 4p 3p 4) silicon 1s 2s 4s 3s 3d 2p 4p 3p 5) iron 6) copper 1s 2s 4s 3s 3d 2p 4p 3p Answer key 1s 2s 4s 3s 2p 4p 3p 3d
What is the orbital diagram for calcium? | Study.com What is the orbital diagram for calcium? Orbital Diagrams: Orbital diagrams are pictorial representations of the arrangement of electrons within the orbitals in an atom or molecule. The orbitals...
Calcium Orbital diagram, Electron configuration, and Valence ... The Calcium orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, the six electrons in 2p orbital, the two electrons in 3s orbital, the next six electrons in 3p orbital, and the remaining two electrons will go in 4s orbital. Orbital diagram for a ground-state electron configuration of a Calcium atom is shown below-
Orbital Filling Diagram For Sulfur Show the orbital-filling diagram for (bromine).Status: Resolved. Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top%(15). 1. Describe the two differences between a 2p x orbital and a 3p y orbital.
PDF 2.Draw Orbital Diagram for following Elements 2.Draw Orbital Diagram for following Elements Elements Orbital filling of Elements Aluminium Calcium Silicon Potassium Chlorine 3.Mark the Electronic Configuration of the elements Magnesium Sodium Fluorine Boron Lithium 9 11 12 5 3
What Is The Electronic Configuration Of Calcium - Usefull ... Under the orbital approximation, we let each electron occupy an orbital the atomic number of calcium is 20. And the electron configuration is the standard notation used to describe the electronic structure of an atom. Calcium Orbital Filling Diagram Source: i.ytimg.com. What Is The Ground State Electron Configuration Of Calcium …. You have to ...
Solved Name Electron Arrangements There are three ways to ... Orbital Filling Diagram (gives the most information) Ex. O2 1s 2s 2p 2. Electron Configuration (quicker to draw than orbital filling diagrams) Ex. O2 152 2s22p 3. Electron Dot shows only the valence (outer energy level) electrons Ex Oxygen atom O: 1.
DOC Electron Configuration Practice Worksheet - Weebly Write orbital filling diagrams, electron configurations, and electron dot diagrams . for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Lewis Dot a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine g. Chromium h. Phosphorus Bromine PAGE THREE INDEPENDENT PRACTICE. Electrons occupy the lowest energy orbital ...
Calcium Orbital Filling Diagram - schematron.org Apr 03, 2019 · Calcium Orbital Filling Diagram 04.03.2019 3 Comments In order to write the Calcium electron configuration we first need to know the we'll put all 20 electrons in orbitals around the nucleus of the Calcium atom. The atomic number of calcium is This means that in a neutral calcium atom, there are 20 protons in its nucleus.
PDF Hund©s Rule & Orbital Filling Diagram Hund©s Rule & Orbital Filling Diagram Complete the orbital diagram for each element. 2) calcium 1s 2s 4s 3s 3d 2p 4p 3p 1) sodium 1s 2s 4s 3s 3d 2p 4p 3p 3) nickel 1s 2s 4s 3s 3d 2p 4p 3p 4) silicon 1s 2s 4s 3s 3d 2p 4p 3p 5) iron 6) copper 1s 2s 4s 3s 3d 2p 4p Answer key 3p 2s2 2p6 3s2 3p6 4s2 1s 2s 4s 3s 2p 4p 3p 3d. Created Date:
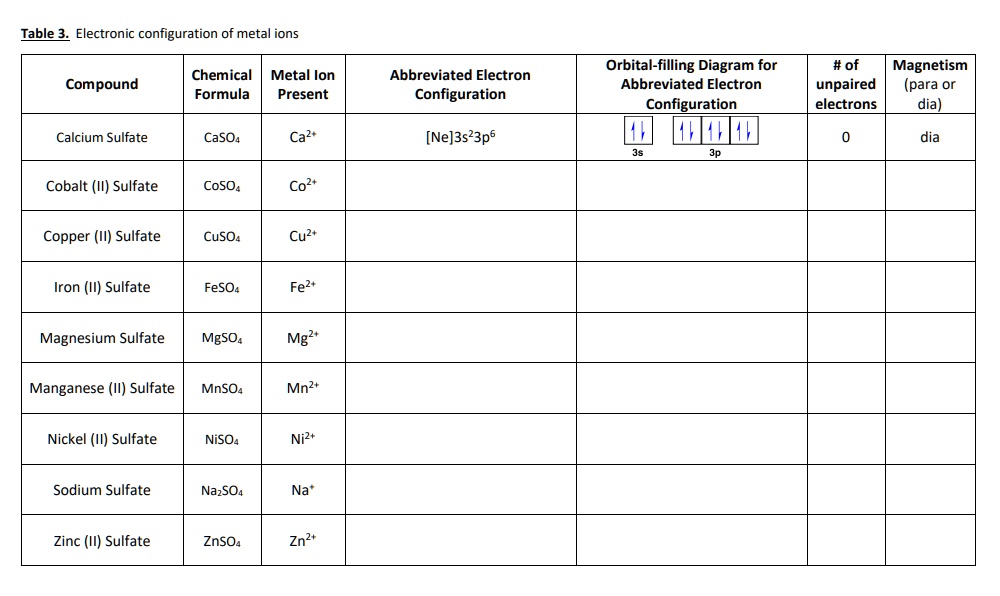
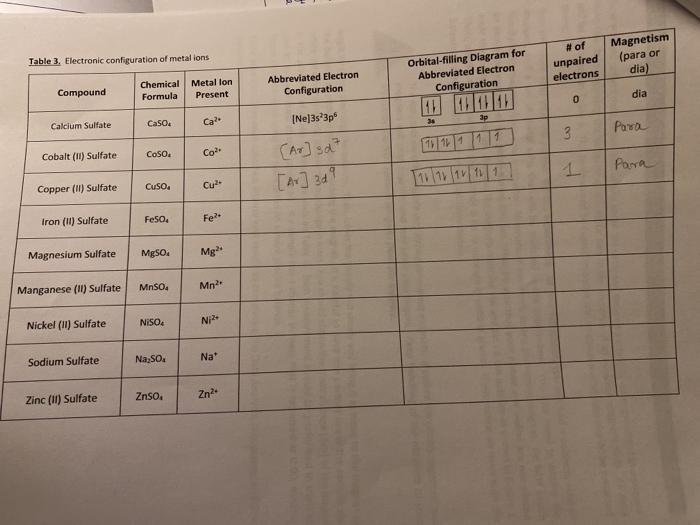
Solved Table 3. Electronic configuration of metal ... - Chegg Question: Table 3. Electronic configuration of metal ions Compound Chemical Metal lon Formula Present Abbreviated Electron Configuration Orbital-filling Diagram for Abbreviated Electron Configuration # of unpaired electrons Magnetism (para or dia) Calcium Sulfate CaSO4 Ca2 [Ne]3s23p6 11111111 0 dia 3s 3p Cobalt (11) Sulfate CoSO4 Co2+ Copper ...
PDF Electron Configurations and Orbital Diagrams key 1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals?
PDF Orbital Filling Diagrams - Oak Park USD Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
Sodium Orbital diagram, Electron configuration, and ... The orbitals are 1s, 2s, 2p, and 3s. The Sodium orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, the six electrons in 2p orbital, and the remaining one electron in 3s orbital. Orbital diagram for a ground-state electron configuration of Sodium atom is shown below-.

:max_bytes(150000):strip_icc()/Calcium-58b602433df78cdcd83d4c16.jpg)







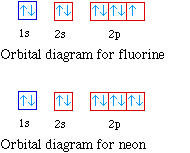











0 Response to "36 orbital filling diagram for calcium"
Post a Comment