36 lewis dot diagram for bromine
How to Draw the Lewis Dot Structure for BrI5: Bromine ... A step-by-step explanation of how to draw the BrI5 Lewis Dot Structure (Bromine pentaiodide).For the BrI5 structure use the periodic table to find the total ... How to draw MgBr2 Lewis Structure? - Science Education and ... The MgBr2 molecule has one central magnesium atom and two bromine atoms. Then the total outermost valence shell electrons can be calculated as follows. ∴ Total outermost valence shell electrons available for MgBr2 Lewis structure ( dot structure) = 2 +2*7= 16 valence electrons in MgBr2. calculation of total valence electron of MgBr2 molecule.
What is the lewis structure of BrO2? - Answers The dot structure for bromine dioxide starts with a Br atom in the center. To the left and right is a singly bonded O atom, each with a pair of dots on the three unbonded sides.
Lewis dot diagram for bromine
How to Draw the Lewis Dot Structure for Br ( the Element ... A step-by-step explanation of how to draw the Br Lewis Dot Structure.For the Br structure use the periodic table to find the total number of valence electron... Lewis Structure Questions and Answers | Study.com Draw the Lewis dot structure for PF5 and provide the following information. a. number of atoms bonded to the central atom b. number of lone electron … Tips for Identifying Intermolecular Forces - Concept ... So, in between the Bromine and the Hydogen, you would have an intermolecular force. So it’s not a bond, like a covalent bond or ionic bond, so it’s not those. The intermolecular force that’s between those two separate atoms, would be a Dipole – Dipole Interaction, because you have opposite or different charges that are interacting between two different molecules. The charges …
Lewis dot diagram for bromine. C2H5OH Lewis Structure, Molecular Geometry, Hybridization ... 2 days ago · Now let us move to the second step of making a Lewis structure. Step 2: Making the electron dot structure. Here the central atom is Carbon which makes Oxygen and Hydrogen the neighboring atoms. Here from the diagram, you can understand how the bond formation takes place for this compound. But let us discuss it in detail for clarity. How to Draw the Lewis Dot Structure for BrI3: Bromine ... A step-by-step explanation of how to draw the BrI3 Lewis Dot Structure.For the BrI3 structure use the periodic table to find the total number of valence elec... Lewis Dot Diagram - Organic Chemistry - Socratic The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is: YouTube. chemistNATE. How to draw BrF3 Lewis Structure? - Science Education and ... It is represented by dots in the BrF3 Lewis diagram. The BrF3 molecule's core bromine atom can be represented as follows: Total outermost valence shell electron of bromine atom in BrF3= 7 Total outermost valence shell electron of fluorine atom in BrF3= 7 The BrF3 molecule has one central bromine and three fluorine atoms.
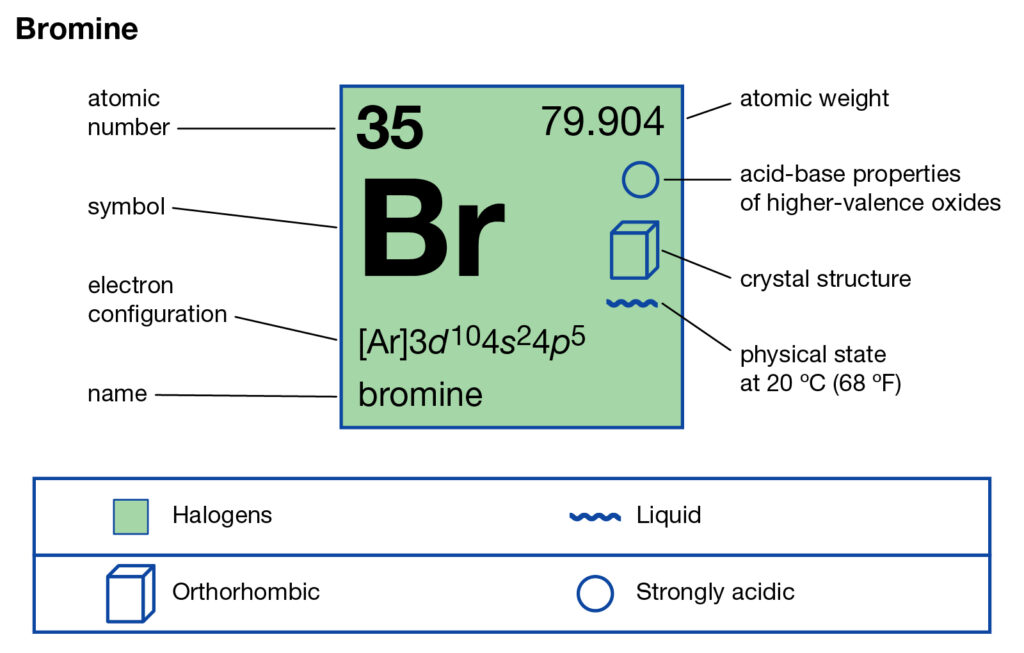
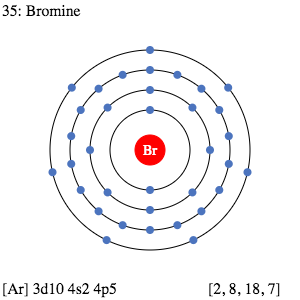
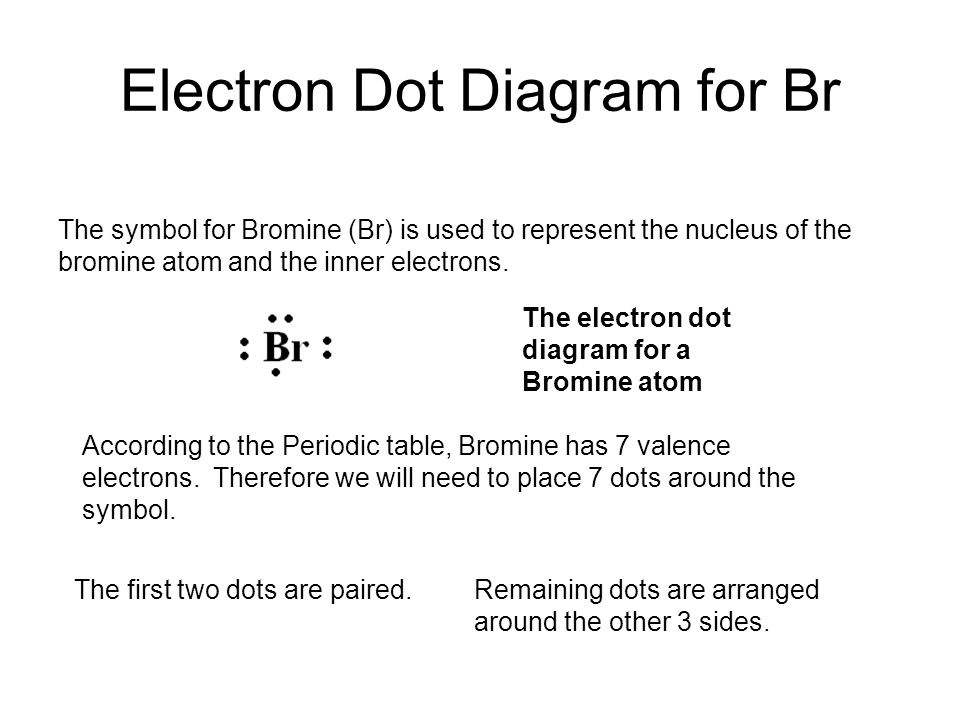
Bromine - Home Bromine Electron Dot Diagram Since Bromine is in group, or series, 17, it has 7 valence electrons. Group 1 on the periodic table has 1 valence electron. Group 2 on the periodic table has 2 valence electrons. The amount of valence electrons in groups 3-12 cannot be predicted because their oxidation numbers are unknown. AP Chemistry 2019 Free-Response Questions - AP Central The complete Lewis electron-dot. diagram for the urea molecule is shown above. (a) Identify the hybridization of the valence orbitals of the carbon atom in the urea molecule. (b) Urea has a high solubility in water, due in part to its ability to form hydrogen bonds. A urea molecule and four water molecules are represented in the box below. Draw ONE dashed line (----) to indicate a possible ... How to Draw the Lewis Dot Structure for Br- (Bromide ion ... A step-by-step explanation of how to draw the Br- Lewis Dot Structure.For the Br- structure use the periodic table to find the total number of valence electr... Br2 Lewis Structure - How to Draw the Lewis Dot Structure ... A step-by-step explanation of how to draw the Br2 Lewis Dot Structure (Bromine gas).For the Br2 structure use the periodic table to find the total number of ...
How to draw SBr2 Lewis Structure? - Science Education and ... To sketch the SBr2 Lewis structure by following these instructions: Step-1: SBr2 Lewis dot Structure by counting valence electrons on the sulfur atom. Step-2: Lewis Structure of SBr2 for counting valence electrons around the terminal bromine atoms. Step-3: Lewis dot Structure for SBr2 generated from step-1 and step-2. What is the Lewis structure for magnesium bromine? - Answers Bromine (Br) has seven valence electrons. This means that the Lewis dot structure has two dots above, below and to the left, and one dot to the right. Lewis Dot Diagrams (Structures) for Atoms and Ions ... Hydrogen / Helium (watch out!) / Bromine Selenium / Nitrogen / Barium Chlorine / Gallium / Argon. WKS 6.2 - LDS for Ions/ Typical Charges. Determine the common oxidation number (charge) for each of the following ions, and then draw their Lewis Dot Structure. Don't forget to show brackets and charge on your LDS for ions! How to draw BrF5 Lewis Structure? - Science Education and ... Key Points To Consider When Drawing The BrF5 Electron Dot Structure. A three-step approach for drawing the BrF5 Lewis structure can be used. The first step is to sketch the Lewis structure of the BrF5 molecule, to add valence electrons around the bromine atom; the second step is to add valence electrons to the five fluorine atoms, and the final step is to combine the step1 and step2 to get the ...
stackfit.us › drawing-ionic-bonds-worksheetstackfit.us Mar 19, 2022 · Draw a Lewis dot diagram for each element listed. H1+ = 1s2 Jan 15, 2021 · Ionic bonding worksheet 1 answers. Name date period type 1 (ionic) bonding worksheet for each pair of elements below draw electron dot structures showing the valence electrons in each atom.
Lewis Structures: Learn How to Draw Lewis Structures ... Bromine, Group 7, has 7 electrons x 2 = 14 Total # of Valence Electrons in MgBr 2 = 16 How to Draw a Lewis Dot Structure Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Example: CO 2 Total = 16 Step 2.
Bromine pentafluoride (BrF5) lewis dot structure ... BrF5 lewis dot structure has 1 bromine and 5 fluorine atom. There is one lone pair present on bromine and it is connected with 5 fluorine atoms with the help of five single bonds. Follow some steps for drawing the lewis dot structure of BrF5. 1. Count total valence electron in BrF5.
[Best Answer] Draw the best lewis structure for bro4- and ... Answer : Lewis-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. The electrons are represented by dot. The given molecule is, perbromate ion. Bromine has '7' valence electrons and oxygen has '6' valence electron.
What is the Lewis dot structure for NH2Cl ... What would a Lewis dot diagram of bromine look like? Since Bromine is in group, or series, 17, it has 7 valence electrons. Group 1 on the periodic table has 1 valence electron. Group 2 on the periodic table has 2 valence electrons. The electron dot diagram for Bromine looks like it does because seven dots are placed in the correct order.
40 bromine lewis dot diagram - Wiring Diagrams Manual Lewis Dot Diagram For Bromine - General Wiring Diagram Lewis electron dot diagrams for ions have fewer for cations or more for anions dots than the corresponding atom. This type of lewis dot structure is represented by an atomic symbol and a series of dots. Any halogen fluorine bromine iodine will have the same electron dot diagram as chlorine.
PDF Lewis Dot Structures Pogil Key - Hudson City School District 9. Draw a Lewis dot diagram for the barium atom. 10. Draw the Lewis dot diagram for the silicon atom. I I, Draw the Lewis dot diagram for the iodine atom. 12. Draw the Lewis dot diagram for the xenon atom. 13. Hypothesize: Why are noble gases considered to be non-reactive? Your group will check your answers with the instructor before moving on.
Chlorine Bohr Model - How to draw Bohr diagram for ... Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Chlorine, we got to know, it has 7 valence electrons. So, just represent the 7 valence electrons around the Chlorine atom as a dot. The electron configuration of Chlorine “Electron configuration is the distribution of electrons of an atom or molecule in …
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more …
CBr4 Lewis Structure - How to Draw the Dot Structure for ... Transcript: This is the CBr4 Lewis structure: Carbon Tetrabromide. Carbon is in group 4 or 14, so it has 4 valence electrons. Bromine in group 7 or 17, so it has 7, and we have 4 Bromines. So 4 plus 28 equals 32 total valence electrons. Carbon, that's the least electronegative, that'll go in the center; and on the outside we'll put the Bromine ...
BrO3- lewis structure, molecular geometry, bond angle ... Lewis diagram is a simple representation of valence electron within a molecule. So, for determining the valence electron in BrO3-, look at the periodic group of bromine and oxygen atoms. By looking at the periodic table, we get to know, bromine belongs to the 17th periodic group and oxygen to the 16th.
BrF3 Lewis Structure (Bromine Trifluoride) - YouTube Hey Guys !In this video we are going to learn the Lewis Dot Structure of Bromine Trifluoride. It has a chemical formula of BrF3 and is made up of one Bromine...
Br2 Lewis Structure: How to Draw the Dot Structure for ... Looking on the periodic table, Bromine is in Group 7 or 17. It has 7 valence electrons. We have two of them though. Multiply that by 2, for a total of 14 valence electrons for the Br2 Lewis structure. First, we'll draw two Bromine atoms next to each other. We have 14 valence electrons for Br2. We'll put two between atoms to form a chemical bond.
Bromine (Br2) Lewis Structure Bromine (Br 2) Molecule Lewis Structure Bromine is a diatomic molecule and contains only bromine atoms. Lewis structure of bromine contains only one Br-Br bond and each bromine atom has three lone pairs. It is very easy to Br 2 lewis structure. Br 2 lewis structure
PBr3 Lewis Structure, Molecular Geometry, Polarity, and ... 2022-03-18 · Lewis Structure is a systematic approach towards deciphering the nature and position of atoms for chemical bonding inside a molecule. This was formulated by Gilbert N Lewis and stands for a diagrammatic representation of bonds and valence electrons of a chemical molecule. Here, we work towards sketching a skeleton diagram of the molecule with atoms …
Boron tribromide (BBr3) lewis dot structure, molecular ... The total number of lone pairs present in the lewis structure of BBr3? The lone pair are represented as dots in the lewis diagram. According to the BBr3 lewis structure, there is a total of 18 dots present (6 dots on each bromine atom). Hence, the number of lone pairs in the BBr3 lewis structure is 9.
Lewis dot diagram for bromine? - Answers What is the Lewis dot diagram for lithium bromine carbon hydrogen silver oxygen iron oxygen potassium bromine and oxygine oxigine? ... the Lewis dot diagram is a table used for the elements, and ...
XeF2 Lewis Structure, Molecular Geometry, Hybridization ... 2021-03-08 · XeF2 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram XeF2 is a covalent inorganic halide formed by the inert gas xenon and the halogen fluorine. This is an active solvent and is found to be soluble in different fluorides like HF …
Lewis Electron Dot Diagrams – Introductory Chemistry – 1st ... A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more …
Carbon tetrabromide (CBr4) lewis dot structure, molecular ... Follow some steps for drawing the lewis dot structure of CBr4. 1. Count total valence electron in CBr4 . Finding the total number of valence electrons in the CBr4 molecule is the first step for drawing its lewis diagram. “A valence electron is the outermost shell electrons around an atom”. To get the valence electron of an atom, look at its periodic group. Since we have to find the …
Tips for Identifying Intermolecular Forces - Concept ... So, in between the Bromine and the Hydogen, you would have an intermolecular force. So it’s not a bond, like a covalent bond or ionic bond, so it’s not those. The intermolecular force that’s between those two separate atoms, would be a Dipole – Dipole Interaction, because you have opposite or different charges that are interacting between two different molecules. The charges …
Lewis Structure Questions and Answers | Study.com Draw the Lewis dot structure for PF5 and provide the following information. a. number of atoms bonded to the central atom b. number of lone electron …
How to Draw the Lewis Dot Structure for Br ( the Element ... A step-by-step explanation of how to draw the Br Lewis Dot Structure.For the Br structure use the periodic table to find the total number of valence electron...

![Answered] Add electron dots and charges as necessary to show ...](https://us-static.z-dn.net/files/d0f/714022937d771ff96cc1c3d4c7b44dcd.jpg)




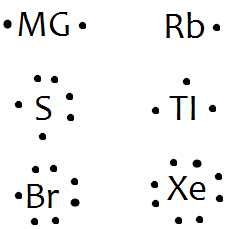


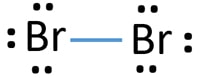











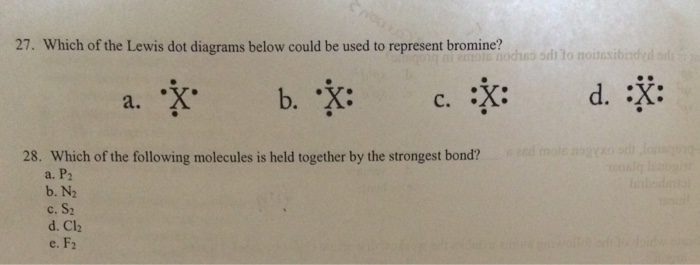




0 Response to "36 lewis dot diagram for bromine"
Post a Comment