38 molecular orbital diagram khan academy
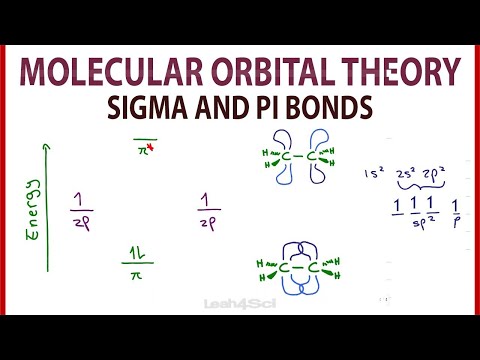
So it's molecular structure looks like this. So you have C double-bonded to C, and then each of those guys have two hydrogens. So let me draw what it would look like, or our best visual, or our best ability to kind of conceptualize what the orbitals around the carbon might look like. So first I'll draw the sp2 hybridized orbitals. This is a very basic introduction to molecular orbital theory. It covers the basics of how to solve for bond order. The intuition of bond order, orbital conf...
So this is how the hydrogen orbital and the carbon orbitals get mixed. The hydrogens 1s orbital bonds with-- well, each of the hydrogen's 1s orbital bonds with each of the carbon's sp3 orbitals. Just so you get a little bit more notation, so when people talk about hybridized sp3 orbitals, all they're saying is, look, carbon doesn't bond.

Molecular orbital diagram khan academy
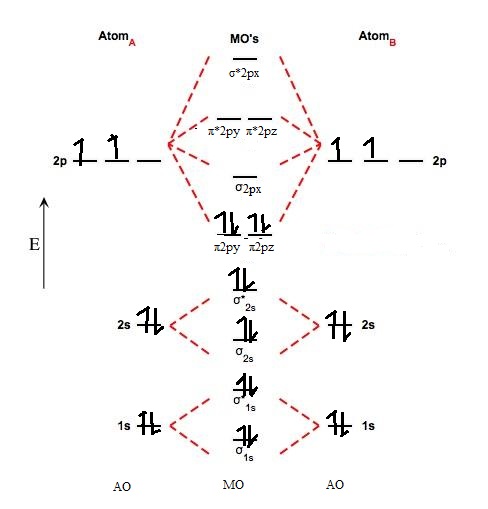
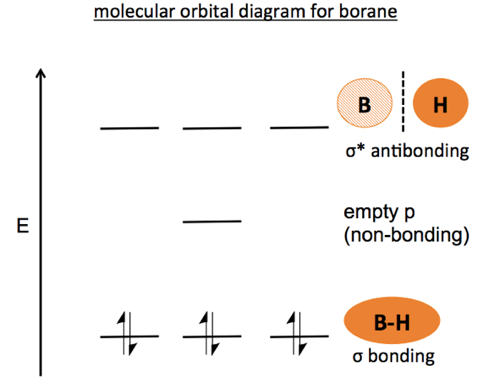
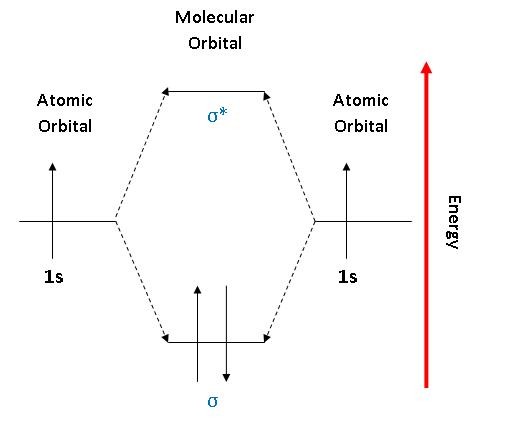
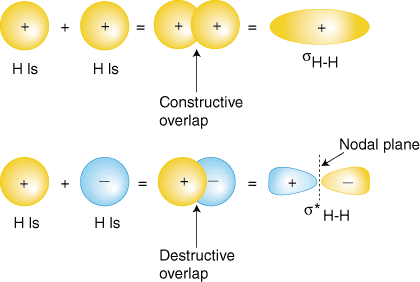
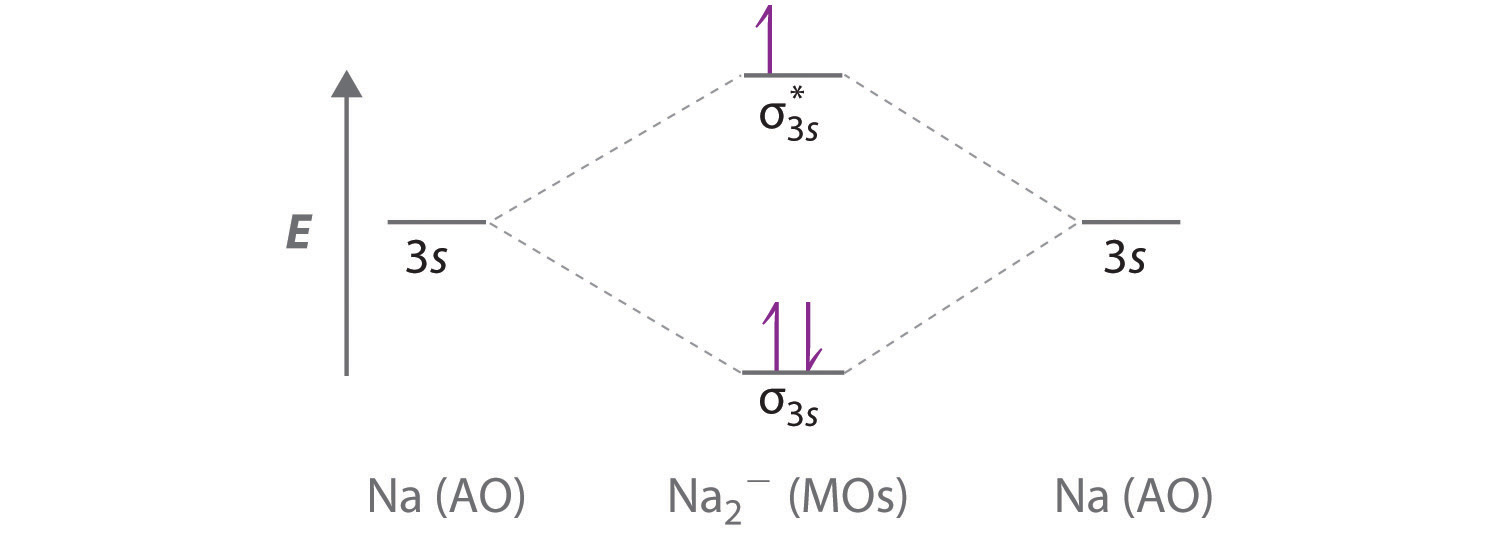
Molecular Orbital Diagrams This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H2+. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic orbitals. sp³ hybridization. In sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character. This type of hybridization is required whenever an atom is surrounded by four groups of electrons. Created by Jay. This is the currently selected item. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram ( Figure 8 ). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons.
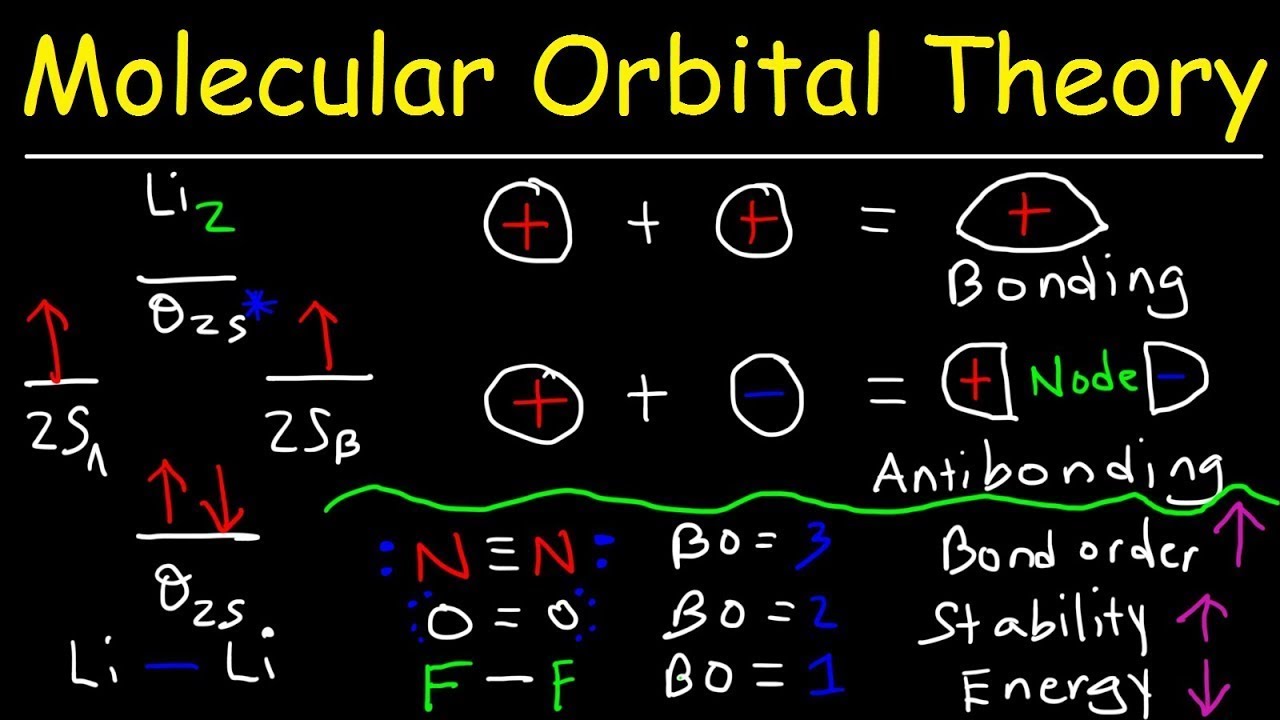
Molecular orbital diagram khan academy. In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each consisting of 50% s character and 50% p character. This type of hybridization is required whenever an atom is surrounded by two groups of electrons. Created by Jay. Google Classroom Facebook Twitter Email Sort by: Tips & Thanks A molecule in which all the electrons are paired, is called diamagnetic while molecule which has one or more unpaired electron is called paramagnetic. Molecular orbital diagram of H2(Hydrogen molecule) : Number of electrons in H2= 2 Electronic configuration of H2= σ1s2 Bond order = 0.5 (Nb-Na) Nb=2 Na=0 B.O = 0.5 (2-0) B.O= 1 1. In molecular orbital theory, a covalent bond is formed whenever two atoms overlap all of their orbitals, regardless of whether they are valence orbitals or not, to create bonding and antibonding orbitals. 2. Yes, this is found in p subshells when forming homonuclear molecules with some atoms. Attention! This video about molecular orbitals is much better: https://www.youtube.com/watch?v=I2k61JMk71MAlright, let's be real. Nobody understands molecula...
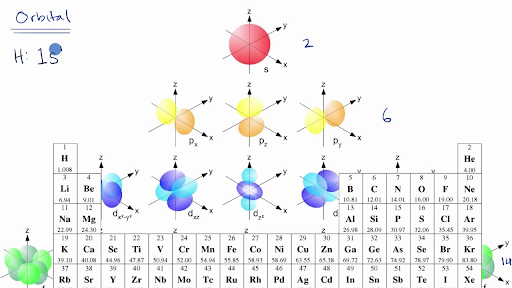
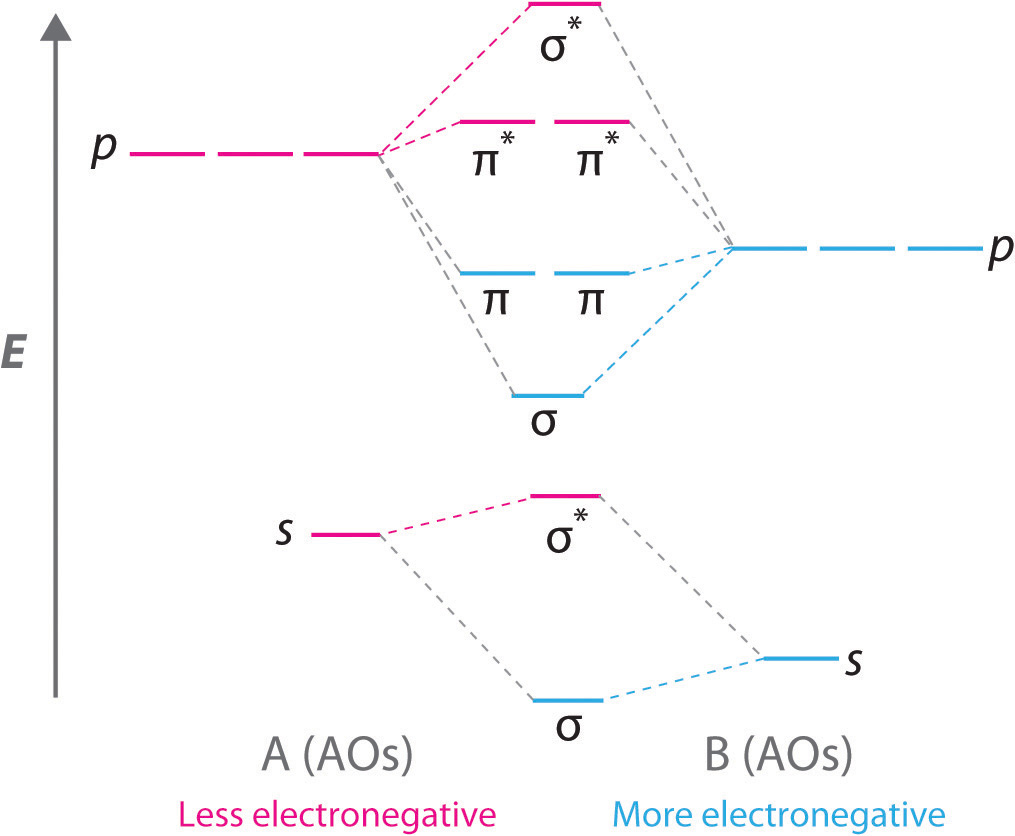
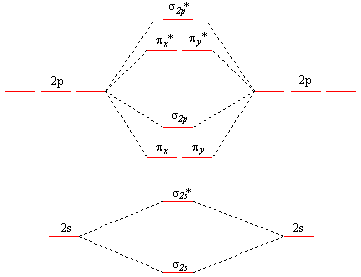
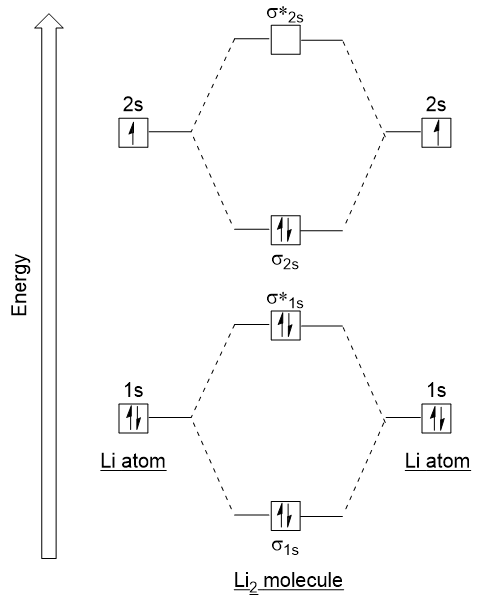
General Notes on Molecular Orbital Diagrams The Y-axis of a MO diagram represents the total energy (not potential nor Gibbs Energy) of the orbitals. Individual atomic orbitals (AO) are arranged on the far left and far right of the diagram. Overlapping atomic orbitals produce molecular orbitals located in the middle of the diagram. •Molecular orbital theory (MO) - a molecule is formed by the overlap of atomic orbitals to form molecular orbitals, electrons are then distributed into MOs. A molecule is a collection of nuclei with the orbitals delocalized over the entire molecule . Dr. Norris discusses frontier molecular orbitals: the HOMO and LUMO. He also discusses applications in understanding reactions and in UV/visible absorption s... molecular orbitals are those formed when valence-shell orbitals are combined. The molecular orbital diagram for an O2molecule would therefore ignore the 1selectrons on both oxygen atoms and concentrate on the interactions between the 2sand 2pvalence orbitals. Molecular Orbitals of the Second Energy Level
The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals. Electrons in the same subshell have the same energy, while electrons in different shells or subshells have different energies. The two 1s orbitals on each Hydrogen atom combine to generate two molecular orbitals - the bonding orbital and the anti-bonding orbital - with energy splitting related to the energy matrix element: 1s r dxˆ Hˆ 1s r dxˆ Vss The total energy is lowered when Hydrogen atoms form a Hydrogen molecule 6 To answer such questions different theories and concepts have been put forward from time to time. In this fourth unit of class 11 chemistry, we can answer the above questions by learning Kössel-Lewis approach, Valence Shell Electron Pair Repulsion (VSEPR) Theory, Valence Bond (VB) Theory and Molecular Orbital (MO) Theory. (Lewis,) VSEPR, Valence Orbitals and MO. In MO theory explaining bonding, anti bonding, and non bonding orbitals in general and how to fill the electrons in the orbitals. Between molecules like N2, O2 and others like HF. Thanks so much!!! oyiebhoynnn816 3 years ago 1 Comment actions Yes please!!! Kananat Siengsano 3 years ago 1 Comment actions
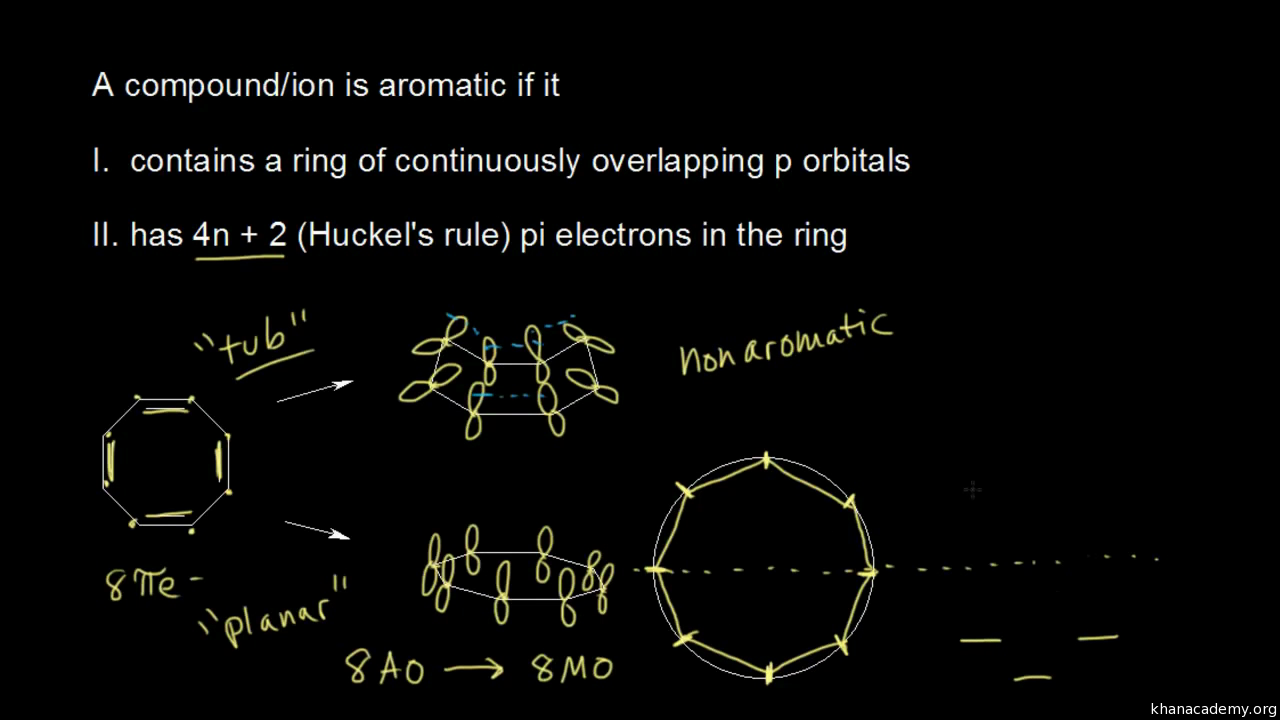
This section illustrates pictorially molecular orbitals for several organic and inorganic molecules. If possible - the energy level diagram is included and clicking upon the relelvant level will generate the accompanying molecular orbital in the right-hand frame. Please choose from: Saturated molecules Molecules with double bonds
Donate here: http://www.aklectures.com/donate.phpWebsite video link: http://www.aklectures.com/lecture/bonding-and-antibonding-molecular-orbitalsFacebook lin...
The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram ( Figure 8 ). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons.
sp³ hybridization. In sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character. This type of hybridization is required whenever an atom is surrounded by four groups of electrons. Created by Jay. This is the currently selected item.
Molecular Orbital Diagrams This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H2+. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic orbitals.

















0 Response to "38 molecular orbital diagram khan academy"
Post a Comment