38 lewis dot diagram for h2s
This question has multiple correct options. Hard. View solution. >. Which statement is true about the most stable Lewis structure for C S 2 ? Medium. View solution. >. Draw an electron dot structure of the ethene molecule. This is the MO diagram of H2S. The left-hand side will contain the atomic orbitals of sulfur i.e 3s2 3px2 3py1 3pz1. And on the right-hand side, there will be atomic orbitals of hydrogen. 8 valence electrons are filled in the MO orbitals. There are two non-bonding orbitals present as well.
Let's do the Lewis structure for H2, Hydrogen gas. It's a quite explosive gas, so please don't fill your blimp up with it. Let's look at the periodic table. Hydrogen is in group 1, that means it has 1 valence electron. But we have 2 Hydrogen atoms, so let's multiply that by 2, for a total of 2 valence electrons.

Lewis dot diagram for h2s
This is the Lewis Dot Structure for H2O. You could alternatively also draw the structure by including two dots for every bond. While oxygen's octet seems to have been filled, hydrogen only has two electrons for its valence shell. Hydrogen and sulfur are both non-metals, and so they SHARE electrons to form covalent bonds (this makes it a covalent aka MOLECULAR compound). Sulfur needs t... The H2 Se Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the H2 Se molecule.
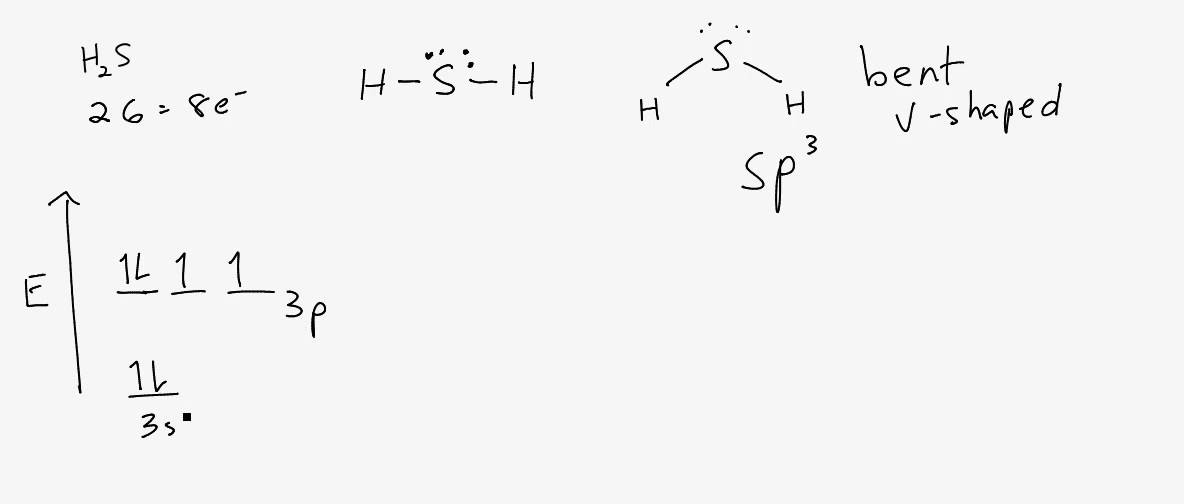
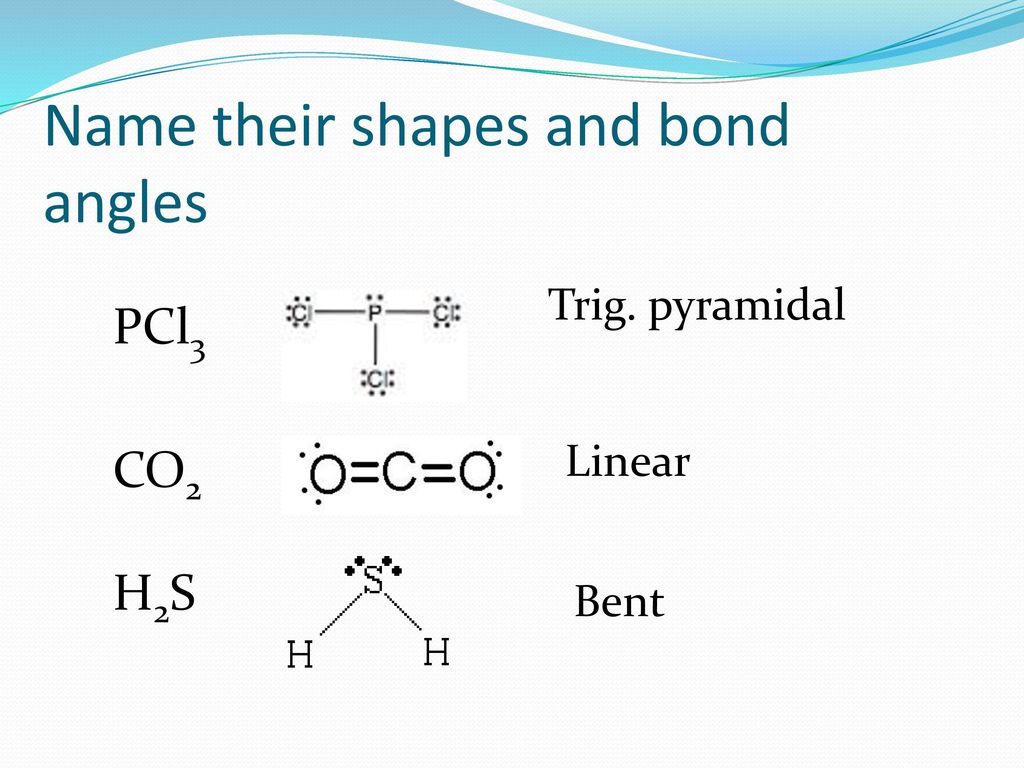
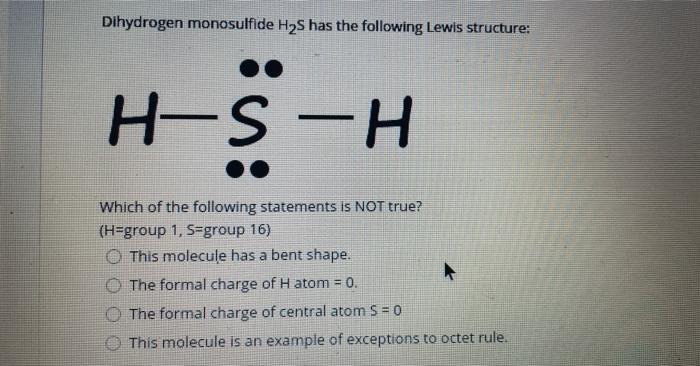
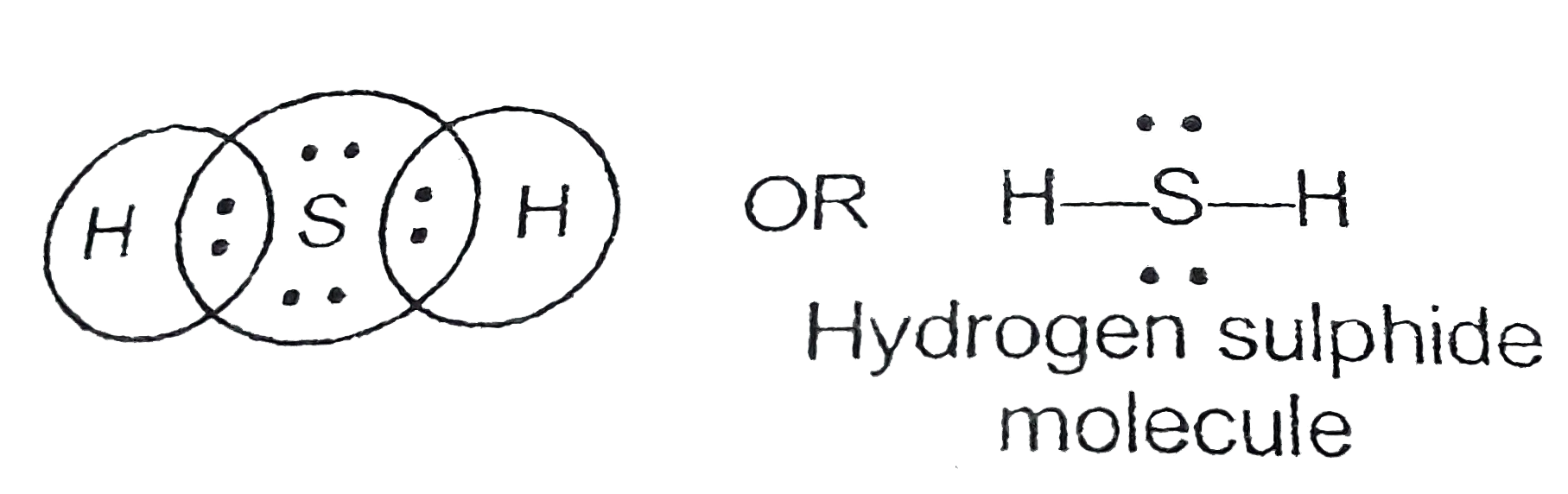
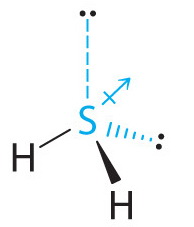
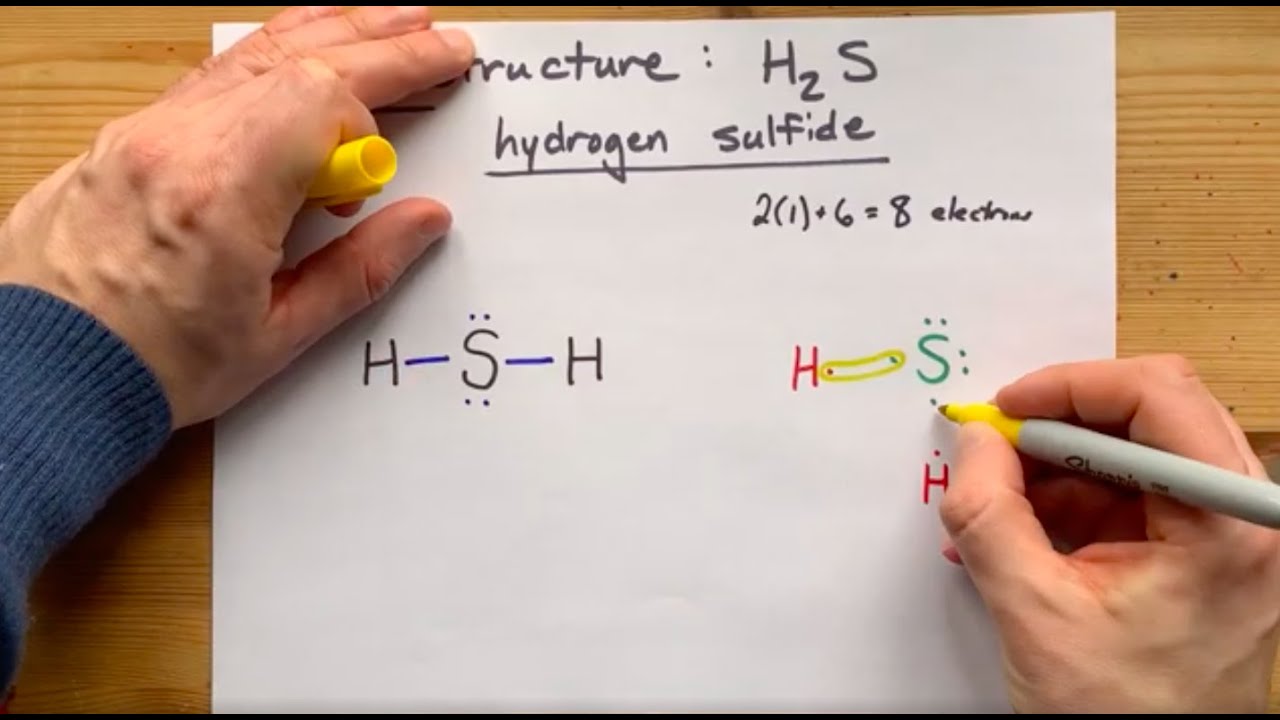
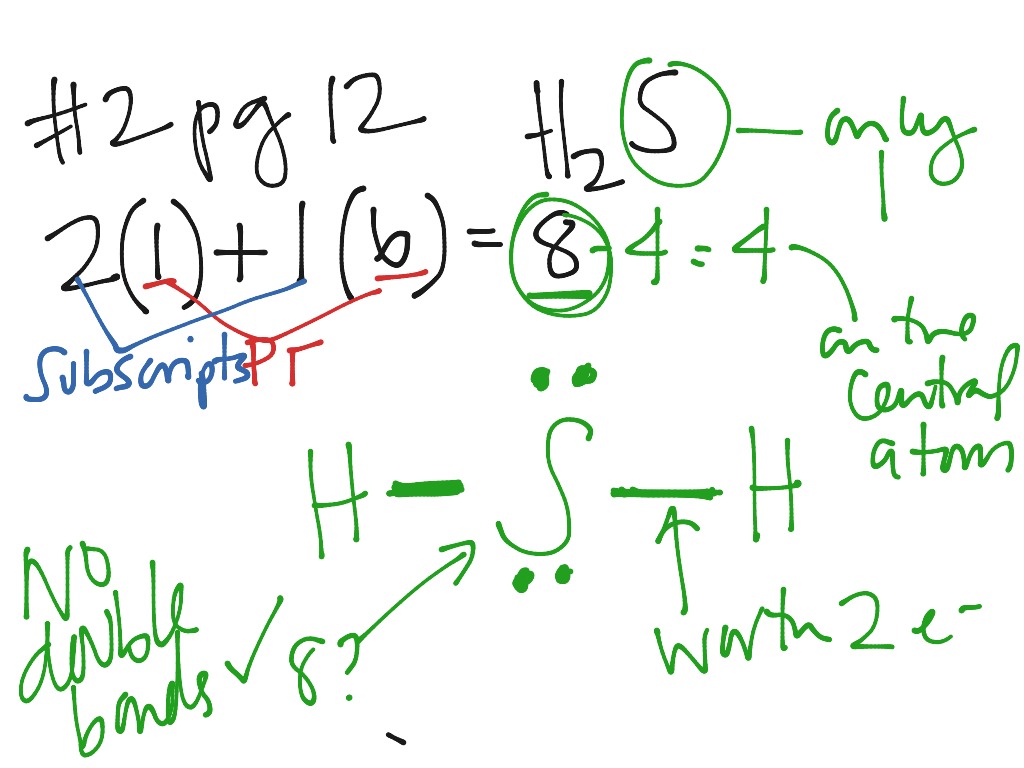
Lewis dot diagram for h2s. 1 Steps for drawing Lewis dot structure of H2S. 2 H2S Molecular Geometry and Shape. 3 H2S Hybridization. 4 Bond Angle of H2S. 5 Is H2S Polar or Non-Polar? 6 H2S MO Diagram. 7 Conclusion. Steps for drawing Lewis dot structure of H 2 S. Count the total number of valence electrons present on each atom of H 2 S molecule. Drawing the Lewis Structure for H 2 S. Viewing Notes: The Lewis structure for H 2 S is very similar to H 2 O. Hydrogen is in Group 1 and therefore has only one valence electron. But since you have two hydrogens you need to multiply by two. Hydrogen atoms only need 2 valence electrons to have a full outer shell. The Lewis structure for H 2 S has a total of 8 valence electrons. Mar 21, 2020 · The Lewis Dot Structure for H2S. Created by MakeTheBrainHappy. This is the Lewis Dot Structure for H2S (hydrogen sulfide). The rules for drawing lewis structures permit the replacement of the bond lines with two electrons. As we have previously discussed, hydrogen only requires two electrons to fill up its valence shell because it only has a 1s shell as the very first element in the periodic table. Hydrogen Sulfide (H2S) Lewis Structure. Lewis structure of Hydrogen sulfide (H 2 S) contains two S-H single bonds around sulfur atom.Also, there are two lone pairs around sulfur atom. Concept of number of total valence electrons of sulfur and hydrogen atoms are used to draw lewis structure of H 2 S. Each step of drawing lewis structure of H 2 S is explained in detail in this tutorial.
The Lewis framework of H2S is similar to H2S. Sulfur needs eight electron to accomplish the requirements for Octet Rule. Yet Hydrogen only requires a solitary electron to end up being stable together it belongs to group 1 elements. Place the Sulphur atom in the middle and arrange that is valence electrons around it. In the H2S Lewis structure diagram, the sulfur atom can be the center atom of the molecule. As a result, central sulfur in the H2S Lewis structure, with all two hydrogen atoms arranged in a tetrahedral geometry. Add valence electron around the hydrogen atom, as given in the figure. Step-3: Lewis dot Structure for H2S generated from step-1 and step-2 A step-by-step explanation of how to write the Lewis Dot Structure for H2S (Dihydrogen Sulfide).The H2S Lewis structure is similar to the structure for water... of two electrons is shown by dots[ ].In H2S Lewis structure,there are two single bonds from sulphur to hydrogens.Besides,the atom sulphur has two lone pairs.Or,In H2S Lewis structure, the atom sulfur follows the octet rule and the hydrogen atoms follow the duet rule.So,H2S molecule follows the octet rule.In H2S Lewis structure,we get four
Complete answer: The Lewis structure of H 2 S is very similar to H 2 O. Hydrogen is in Group 1 and therefore has only one valence electron. Hydrogen atoms only need 2 valence electrons to have a full outer shell. The Lewis structure for H 2 S has a total of 8 valence electrons. Hydrogen has 1 valence electron, but we have two Hydrogens here. Lewis's structure of SH2 is really helpful to determine its electron geometry, molecular shape, number of shared pair, and lone pair electrons. Follow these steps to draw the lewis dot structure for H2S. Step 1: In the first step, determine the total valence electron present in H2S. As we know hydrogen only has one valence electron in its ... Lewis dot Structure of H2S The chemistry graduates often encounter the hydrogen sulfide molecule due to the wide range of industrial applications. If we look into the formation of hydrogen sulfide molecules only two bonds of. If your student asks about the H2S Lewis Structure both hydrogen sulfide gas is colourless gas smell like rotten eggs ... To draw the lewis dot structure of H₂S, we have to find out the valence electrons of sulfur and hydrogen first.We express valence electrons as dots in lewis dot structure. To get the valence electrons of sulfur,we need to look at the electronic configuration of sulfur. S (16)=1s²2s²2p⁶3s²3p⁴. More ›.

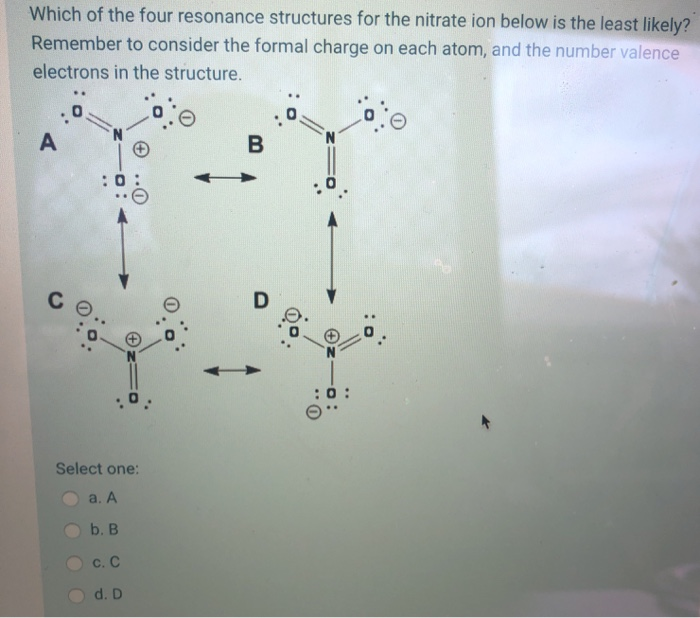
Today’s Do Now Write the Lewis dot diagram for the ionic bond between sodium and sulfur. Write the Lewis structure for the H2S molecule. Do resonance.
The Lewis Structure of Hydrogen Sulfide is easy to draw and understand. In this compound, both the hydrogen atoms require one electron to make the covalent bond with Sulfur. The Lewis structure of H2S is similar to H 2 S. Sulfur needs eight electrons to fulfill the requirements for Octet Rule. But Hydrogen only requires a single electron to ...
H2s Lewis Dot Diagram. Here are a number of highest rated H2s Lewis Dot Diagram pictures on internet. We identified it from reliable source. Its submitted by admin in the best field. We recognize this nice of H2s Lewis Dot Diagram graphic could possibly be the most trending subject as soon as we share it in google plus or facebook.
Hydrogen Sulfide Lewis Dot Structure For H2s. Here are a number of highest rated Hydrogen Sulfide Lewis Dot Structure For H2s pictures upon internet. We identified it from obedient source. Its submitted by meting out in the best field.
The H2 Se Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the H2 Se molecule.
Hydrogen and sulfur are both non-metals, and so they SHARE electrons to form covalent bonds (this makes it a covalent aka MOLECULAR compound). Sulfur needs t...
This is the Lewis Dot Structure for H2O. You could alternatively also draw the structure by including two dots for every bond. While oxygen's octet seems to have been filled, hydrogen only has two electrons for its valence shell.














_1637872200.bmp)







![Expert Verified] 5 Draw the electron dot structures for (a ...](https://hi-static.z-dn.net/files/da2/381a12f3bead92c828a3692766ca8560.png)


0 Response to "38 lewis dot diagram for h2s"
Post a Comment