40 orbital diagram for titanium
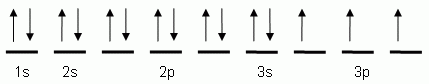
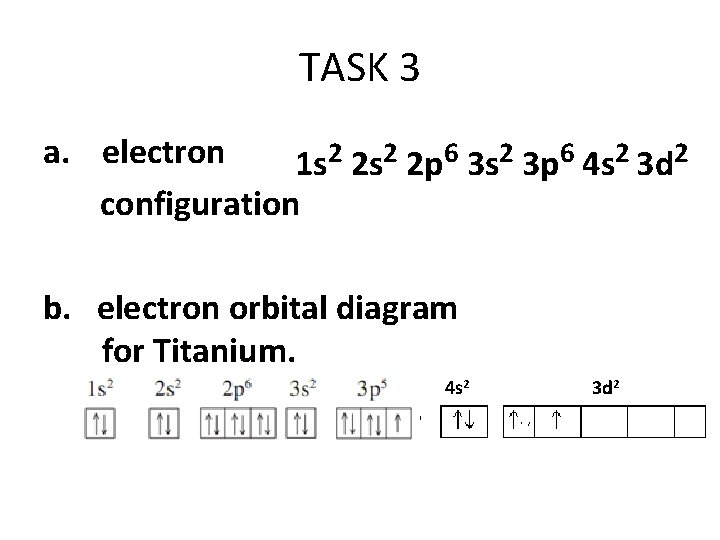
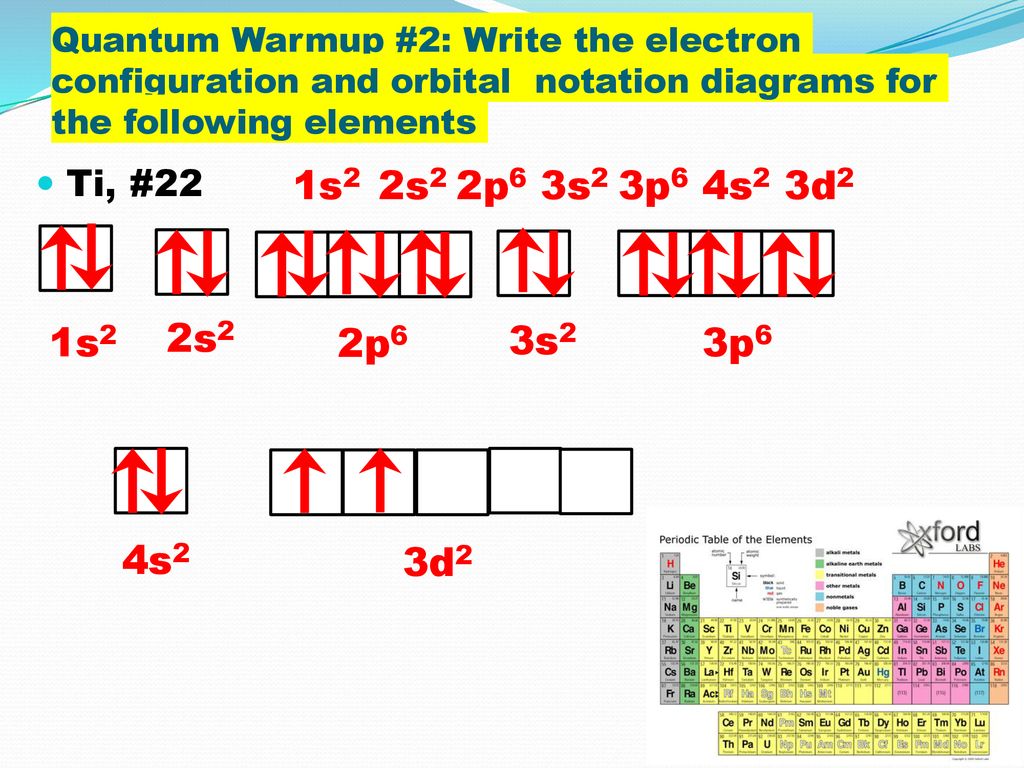
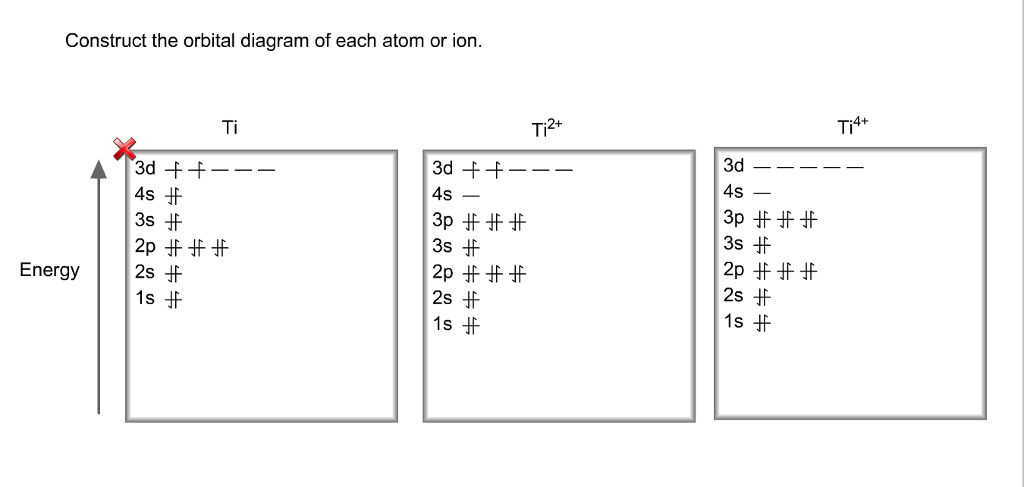
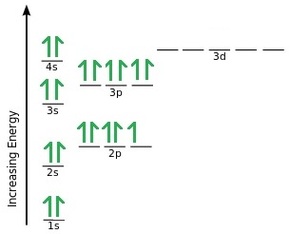
Let's consider titanium (Z = 22). Its electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 2, which the (n + l) rule correctly predicts. If the electron configuration depended solely on the orbital energies, we would expect: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 4 - with no electrons in the 4s orbital. Draw and explain the orbital diagram for titanium. Transition Metals: There are, in total, four transition series of the d-block elements starting with 3d, 4d, 5d and 6d respectively.
Titanium electron configuration. Ti (Titanium) is an element with position number 22 in the periodic table. Located in the IV period. Melting point: 1660 ℃. Density: 4.51 g/cm 3 . Electronic configuration of the Titanium atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 2. Electronic configuration of the Titanium ...

Orbital diagram for titanium
The ground state electron configuration of ground state gaseous neutral titanium is [Ar].3d2.4s2 and the term symbol is 3F2. Kossel shell structure ... This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble... The electron configuration for titanium is 1s22s22p63s23p63d24s2, according to the Jefferson Lab website. The element’s 22 electrons are arranged in four energy levels surrounding the nucleus of the atom. Electrons orbit the nucleus in energy levels, which are also called shells.
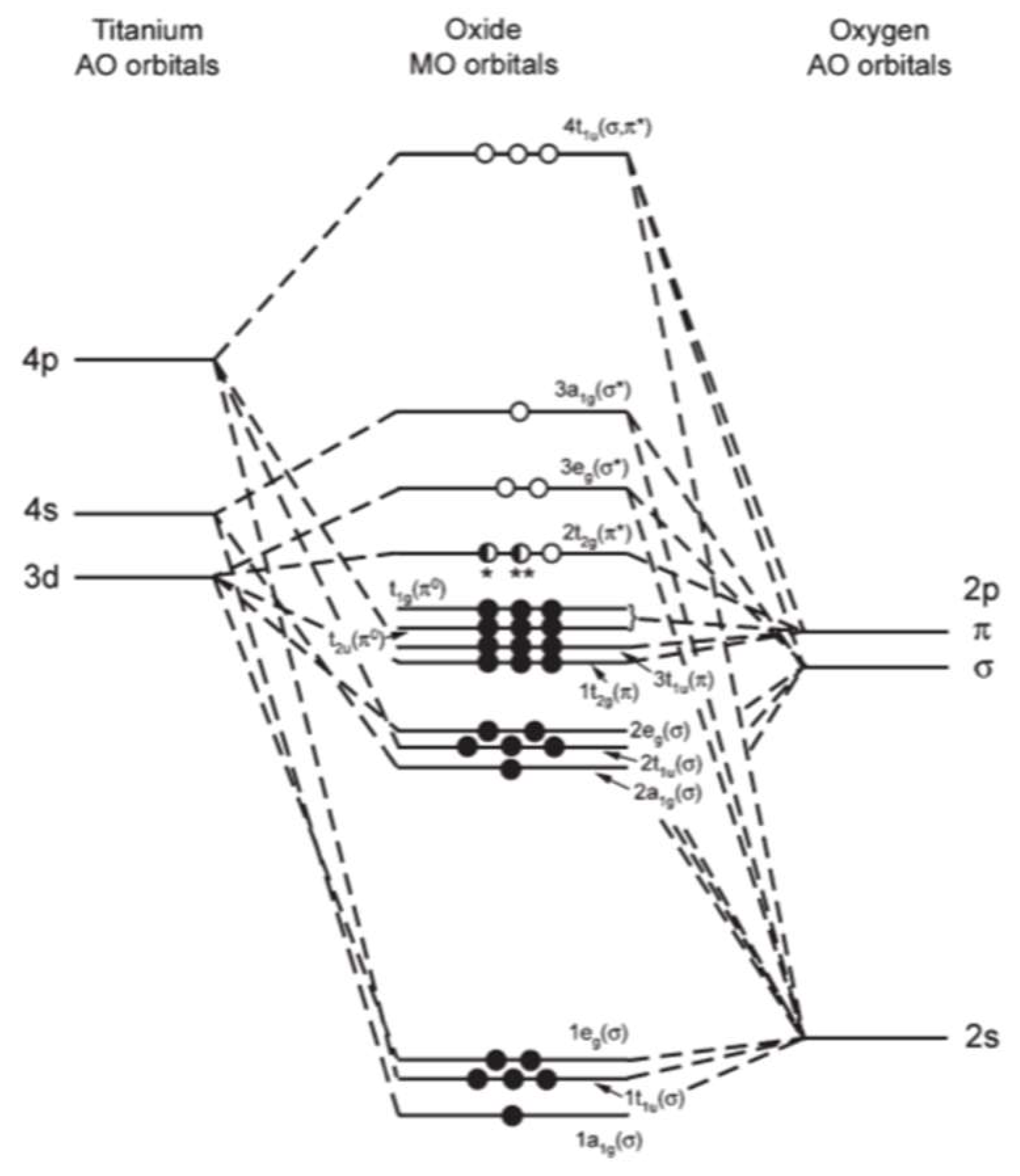
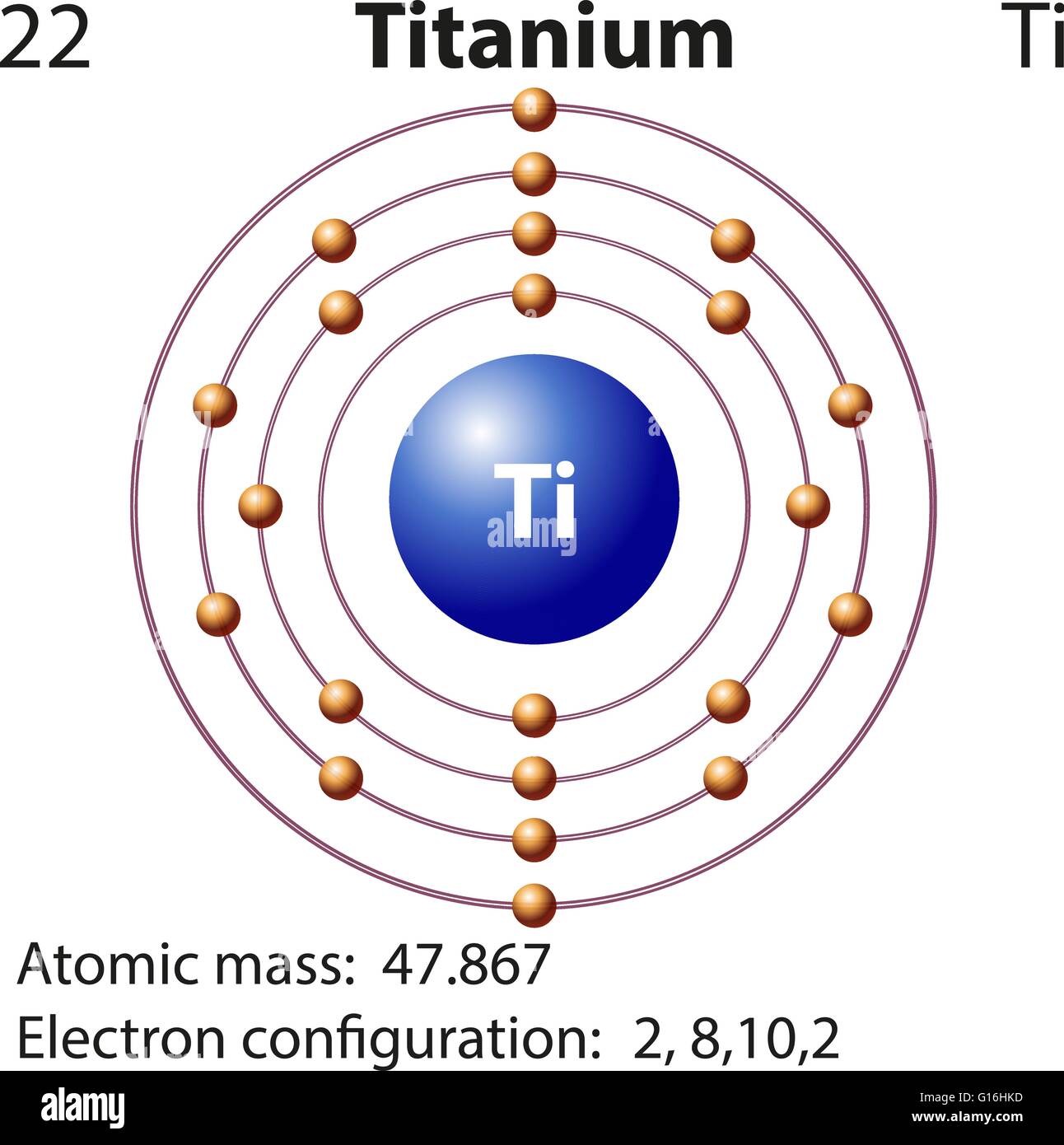
Orbital diagram for titanium. An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s. To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti). We first need to find the number... Interaction Diagram. The molecular orbitals of the two extreme geometries 2 and . 4 . are constructed in Figure . 3. In the middle of the figure, there are four d-block orbitals of each Ti fragment, r = 0.2 . A . and . r = 0.0 . A. The . yz . orbital is high above in energy due . to . the strong ligand field of (O,,),, and is not shown. For . r ... For that, we have electron shell diagrams. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. For each electron shell atom diagram, the element symbol is listed in the nucleus. This is a Bohr Diagram of a titanium atom. The nucleus of a titanium atom has 22 protons and 26 neutrons.
This means that when titanium loses electrons, it does so from the 4s orbital first. Ti: 1s22s22p63s23p63d24s2 Therefore, the two electrons that are lost when the Ti2+ is formed will come from the 4s orbital, which means that the electron configuration of the cation is orbital #4s, so you can see here However, once the #4s # orbital is filled, it becomes higher in energy than the orbital#3d #. This means that when titanium loses electrons, it does so from #4s # orbital first. #Ti: 1s^2 2s^2 2p^6 3s^2 3p^6 3d^2 4s^2# Therefore, the two electrons that get lost when The electron configuration for titanium is [Ar]3d24s2. The two 3d electrons would be placed in two separate orbitals, so there are two half filled 3d orbitals in a titanium atom. Jun 12, 2018 · Draw the orbital diagram for the d orbitals in an octahedral complex containing. Time-saving video by Brightstorm on Electron Configurations for Transition Metals and Their Ions Problem.The base expression (ground state; Ti0) is [Ar]3d24s2 so removal of 2 electrons to get the oxidation state of 2+ would result in the configuration of [Ar] 3d2.
Electron Configuration Of Selenium - 9 images - please answer asap question use the periodic table to, flw incorporated specialists in physical measurement, Energy level diagram. So, first off, all we discuss about the reluctantly configurations or titanium three plus is equal toe here. Argon and the street. Even that Minniti orbital contained one electron. And here argon is ating 18 plus 1 19 left contentment 1911 are involved in that in m three. Plus I'm. And for titanium, There is 20 to let Tom. Orbital Diagram. 1s ... Titanium dioxide (TiO2), a white pigment that covers surfaces very well, is used in paint, rubber, paper and many others. Sources Usually occurs in the minerals ilmenite (FeTiO3) or rutile (TiO2). Also in Titaniferous magnetite, titanite (CaTiSiO5), and iron ores. Pure metal produced by heating TiO2 with C and Cl2 to ... The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...
Orbital diagram for titanium(Ti) Titanium(Ti) excited state electron configuration. Atoms can jump from one orbital to another orbital by excited state. This is called quantum jump. Ground state electron configuration of titanium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 2 4s 2. The valency of the element is determined by electron configuration in the excited state.
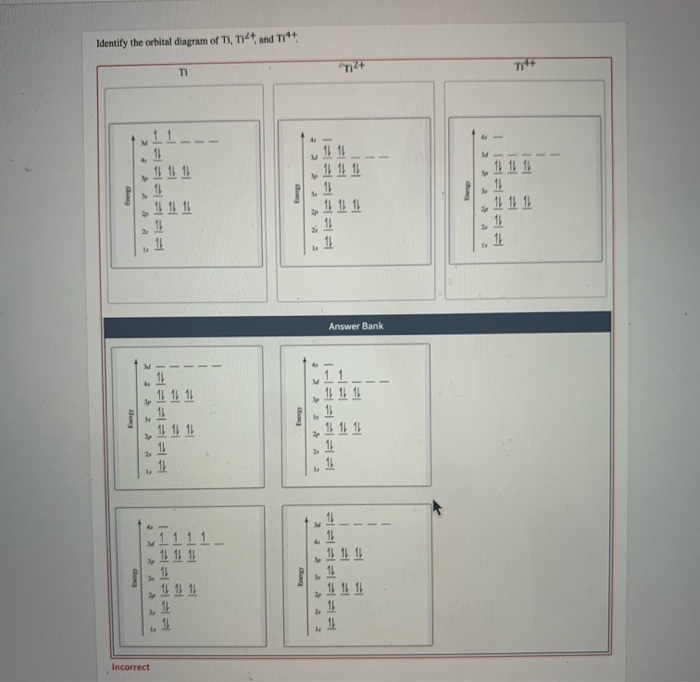
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (10 ratings) Transcribed image text: Construct the orbital diagram of each atom or ion. What is the electron configuration for titanium?
Titanium Electron Configuration (Ti) with Orbital Diagram January 26, 2021Leave a Comment Titanium Electron Configuration: Titanium is a chemical element that has a chemical symbol Ti. Its atomic number is 22. It is a transition metal lustrous which has a silver colour, low density, and high strength.
Molecular orbital diagram of ticl4 Titanium tetrachloride Names IUPAC Name Titanium (IV) Chloride Other Names Titanium Tetrachloride Tetrachlorotitanium Identifiers CAS Number 7550-45- Y 3D model (JSmol) Interactive image ChemSpider 22615 Y ECHA InfoCard 100.028,584 EC Number 231-441-9 MeSH
Row 4: K p -V sys diagram showing the expected and observed orbital and radial velocities. In the case of different velocities, the observed velocity is indicated with an opaque line.
The electron configuration of titanium and the orbital diagram is the main topic in this article…. Read More Titanium(Ti) electron configuration and orbital diagram. Arsenic(As) electron configuration and orbital diagram. Arsenic(As) is the 33rd element in the periodic table and its symbol is 'As'. The electron configuration of arsenic ...
Titanium is a metal. It's not just any metal, it's a transition metal. Being a transition metal, it has a special electron configuration. It adds its next electron to the third shell, not the outermost fourth shell. With a configuration of 2-8-10-2, titanium is out in the world and ready to bond with other elements.
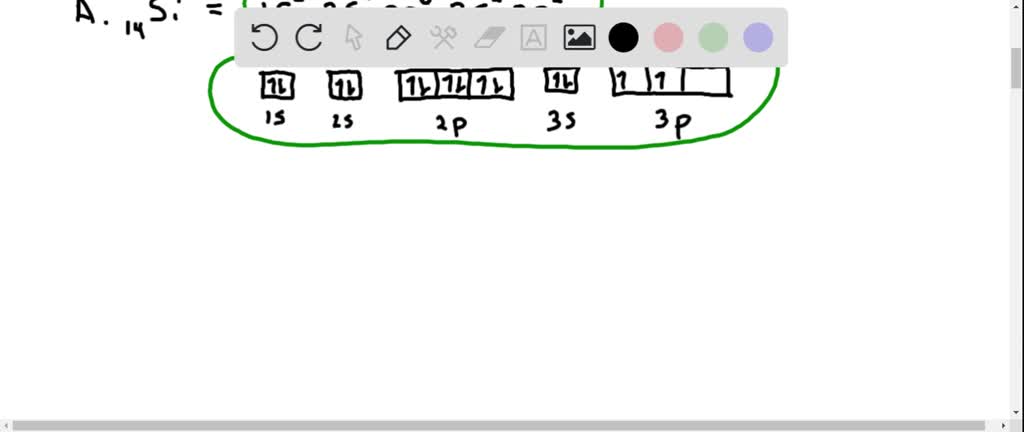
according to hunds rule and the aufbau principle the electron configuration for the titanium atom can best be expressed as 33903
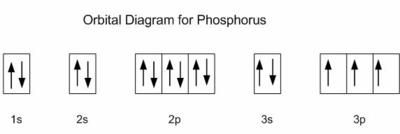
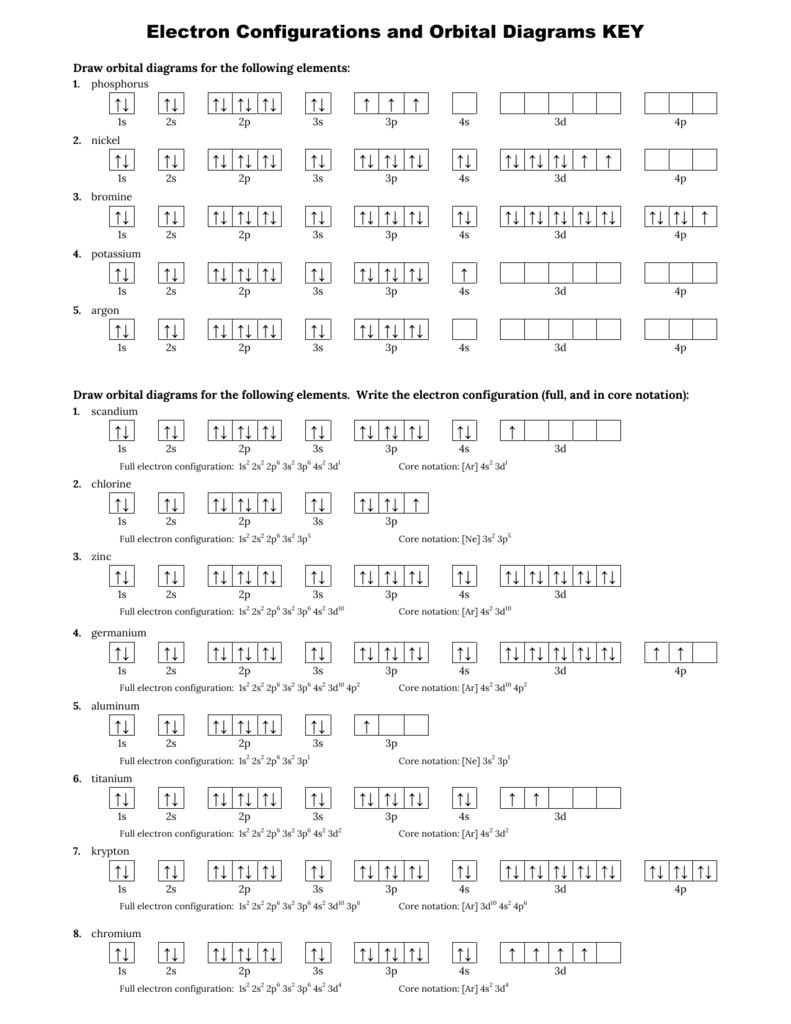
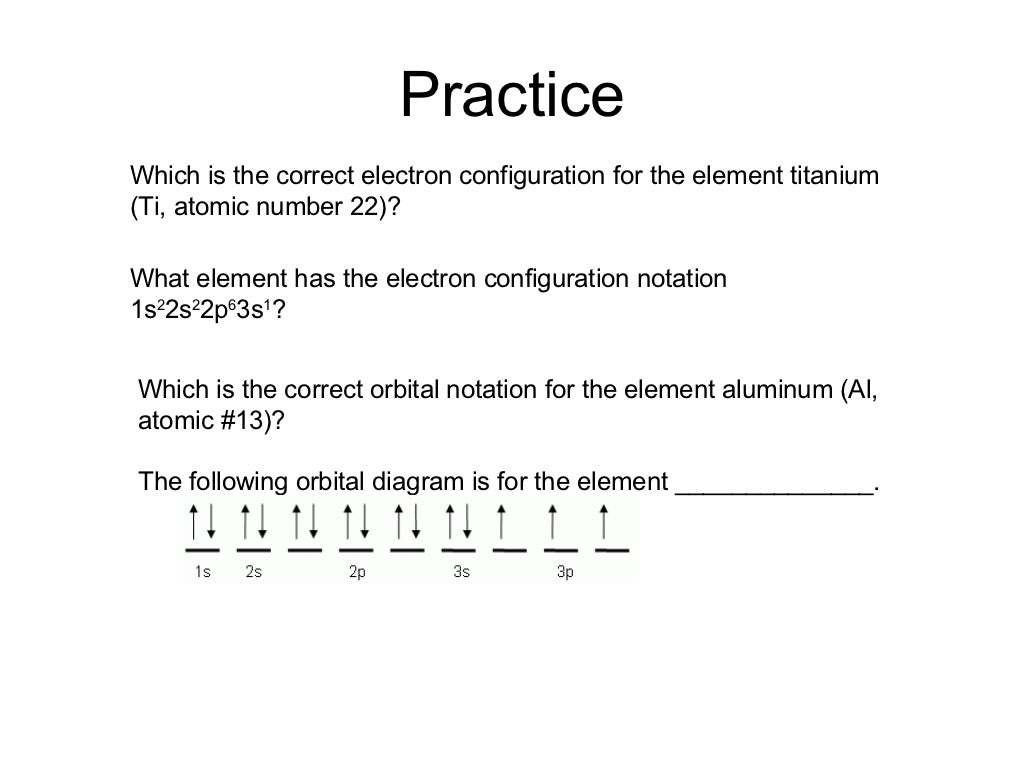
Draw the orbital diagram for the following elements: Oxygen (O) Titanium (Ti) Silicon (Si) Copper (Cu) For each of the following elements, identify if the electron configuration is correct or incorrect. If it is incorrect, give the fix to the configuration. Carbon (C) = 1s22s22p2. Sulfur (S) = 1s22s22p63p6
Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31:
The electron configuration for titanium is 1s22s22p63s23p63d24s2, according to the Jefferson Lab website. The element’s 22 electrons are arranged in four energy levels surrounding the nucleus of the atom. Electrons orbit the nucleus in energy levels, which are also called shells.
This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble...
The ground state electron configuration of ground state gaseous neutral titanium is [Ar].3d2.4s2 and the term symbol is 3F2. Kossel shell structure ...





















0 Response to "40 orbital diagram for titanium"
Post a Comment