37 what is orbital diagram
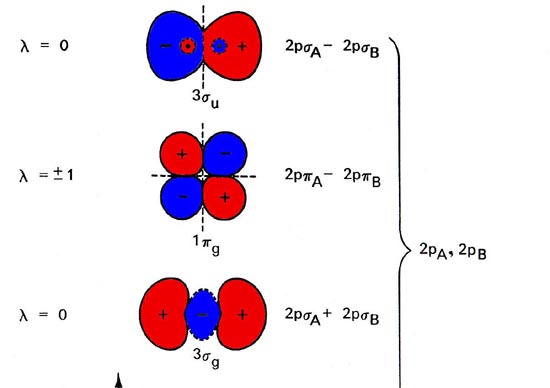
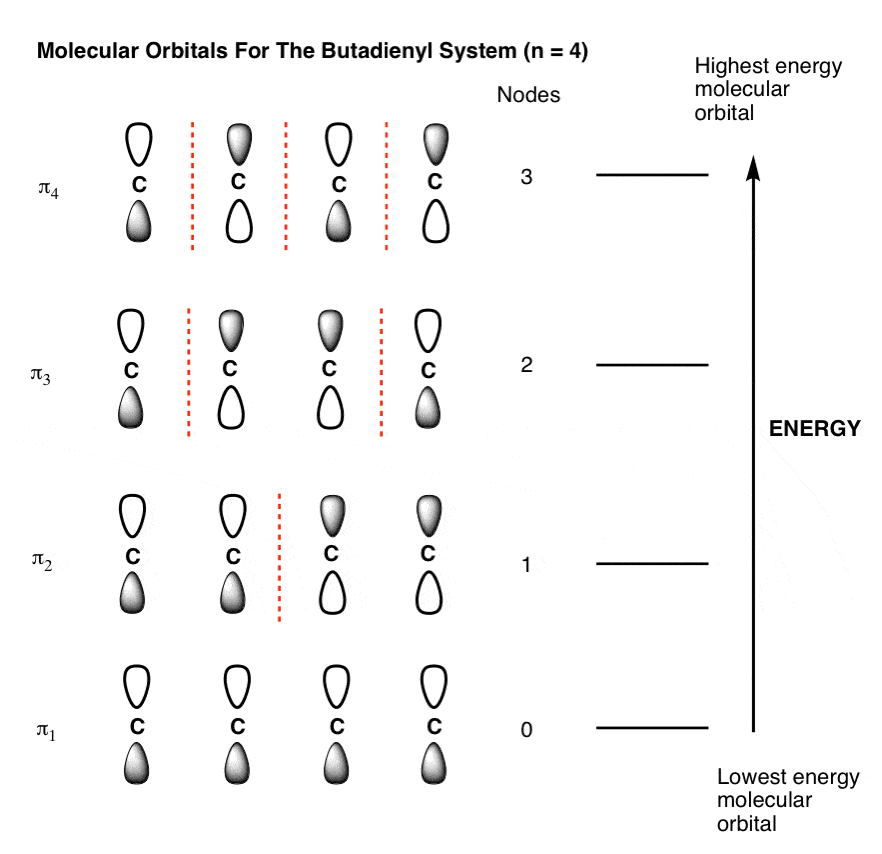
Table Partial Orbital Diagrams and Electron Configurations * for the Elements in Period 4. * Colored type indicates the sublevel to which the last electron is added. Figure A periodic table of partial ground- state electron configurations. Figure Write orbital diagram for Au+. Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
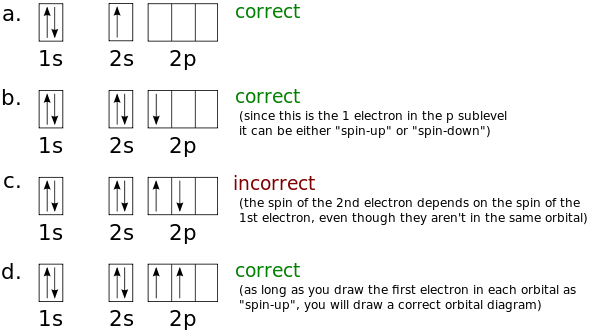
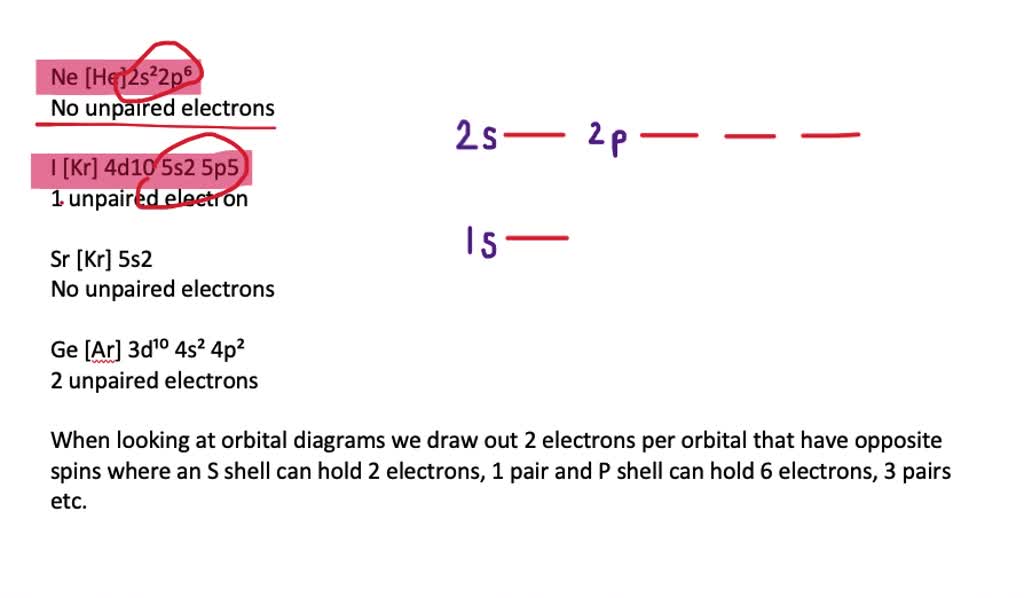
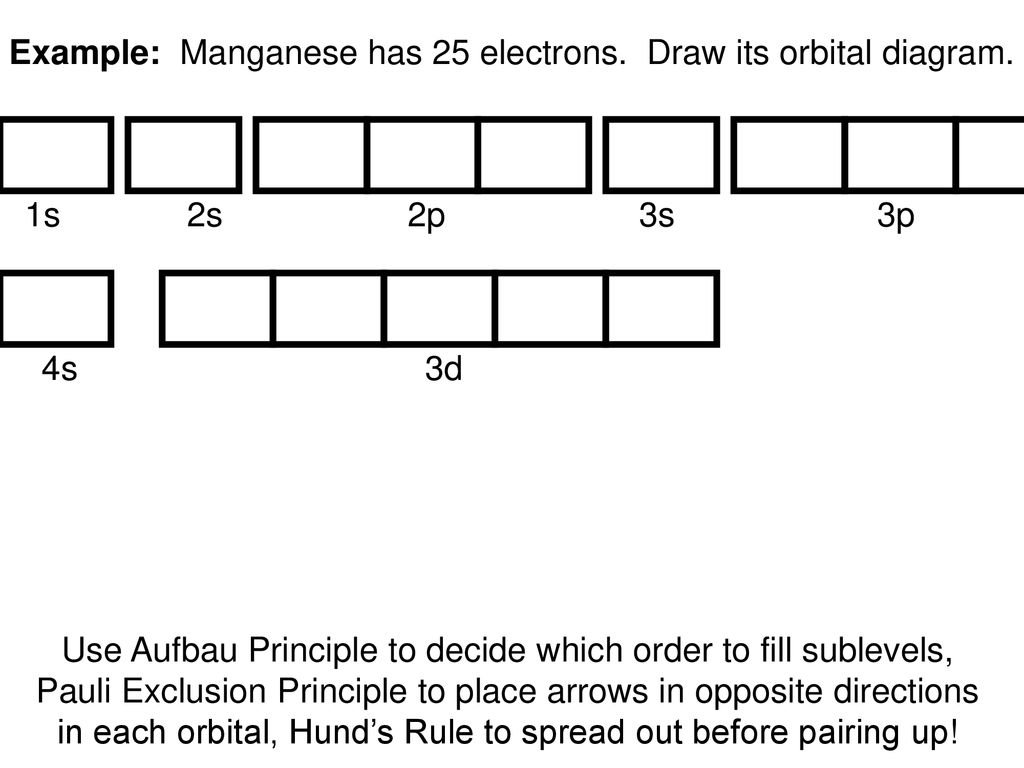
Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. The Pauli Exclusion Principle says that only two electrons can fit into an single orbital.

What is orbital diagram
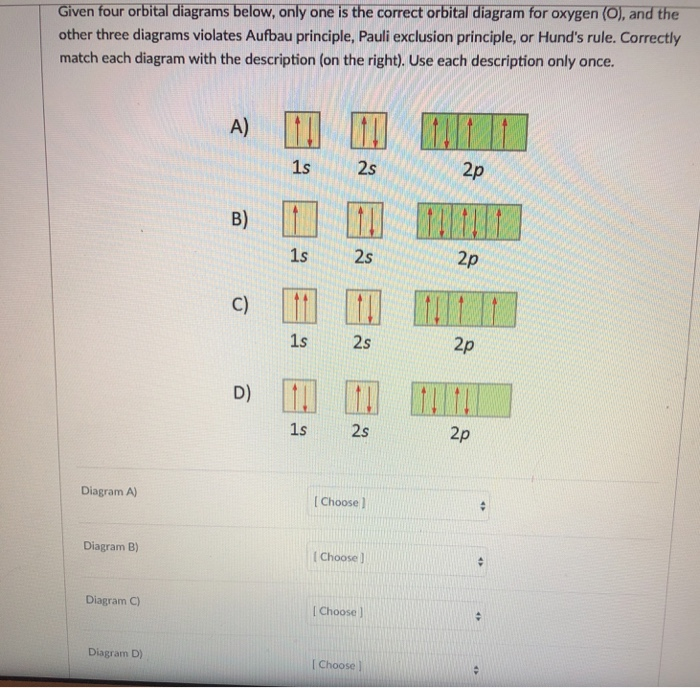
What is incorrect about this orbital diagram? * 1s 2s 2p O Both arrows in the filled 2p box should be pointing the same direction O All the arrows should be pointing the same direction. O There is nothing incorrect with this diagram O In the there should only be 1 arrow in the first 2p box and one in the 2nd 2p box. check_circle. What is the orbital diagram for Mo? A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. What is the MO configuration for b2? Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).
What is orbital diagram. 8 - Drawing Molecular Orbital Diagrams. Abstract (TL;DR) Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already understand about sigma and pi bonds. Depending on if it is a homonuclear case, where the bonding atoms are the same, or a heteronuclear case, where the bonding atoms are ... What is the orbital diagram of CO 27? In this case of Cobalt there are 27 electrons which are present in 4 orbits and their distribution on the orbit that is electronic configuration can be written as: 1s22s22p63s23p63d74s2. The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org! Arsenic atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. What is Orbital Diagram 3. What is Electron Configuration 4. Side by Side Comparison - Orbital Diagram vs Electron Configuration in Tabular Form 6. Summary. What is Orbital Diagram? The orbital diagram is a type of diagram which shows the distribution of electrons in the orbitals of an atom and indicates the spin of those electrons.
Atomic Orbital Diagram for Iron(Fe) Iron ion(Fe 2+,Fe 3+) electron configuration. Ground state electron configuration of iron(Fe) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2.The electron configuration shows that the last shell of iron has two electrons and the d-orbital has a total of six electrons. What is a orbital diagram? Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. Orbital diagrams are a pictorial description of electrons in an atom. What is a molecular orbital (MO)? This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... Selenium atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. An orbital diagram uses boxes with arrows to represent the electrons in an atom. Each box in an The following is an orbital diagram for selenium. In writing an.
Orbital diagrams give you all of the information you need about the electron configuration and occupied spin states for chemistry or physics, and are easy to both create and interpret. Sciencing_Icons_Science SCIENCE Sciencing_Icons_Biology Biology Sciencing_Icons_Cells Cells Sciencing_Icons_Molecular Molecular Sciencing_Icons_Microorganisms A p orbital has the approximate shape of a pair of lobes on opposite sides of the nucleus, or a somewhat dumbbell shape. An electron in a p orbital has equal probability of being in either half. What do orbital diagrams show? An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. The Pauli Exclusion Principle says that only two electrons can fit into an single orbital. Additionally, what is the orbital diagram for iron? Video: Fe, Fe 2 +, and Fe 3 + Electron Configuration Notation Since 1s can only hold two electrons the next 2 electrons for Iron go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. The Pauli Exclusion Principle says that only two electrons can fit into an single orbital.
Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau...
Orbital filling diagrams essentially just turn this big list of electron locations into a picture that shows not just what type of electrons an orbital resides in, but also which of those orbitals they're located in. Don't worry - this is easier than it seems. The rules for orbital filling diagrams
The standard atomic mass of sodium is 22.989769. The period of sodium is 3 and it is a s-block element. This article gives an idea about the electron configuration of sodium(Ne) and orbital diagrams, period and groups, valency and valence electrons of sodium, bond formation, compound formation, application of different principles.
Orbital diagrams are pictorial descriptions of the electrons in an atom. The Pauli Exclusion Principle says that only two electrons can fit into an single orbital. Hund s rule states that electrons go into different orbitals in the same sub-level before doubling up inside orbitals. Click to see full answer
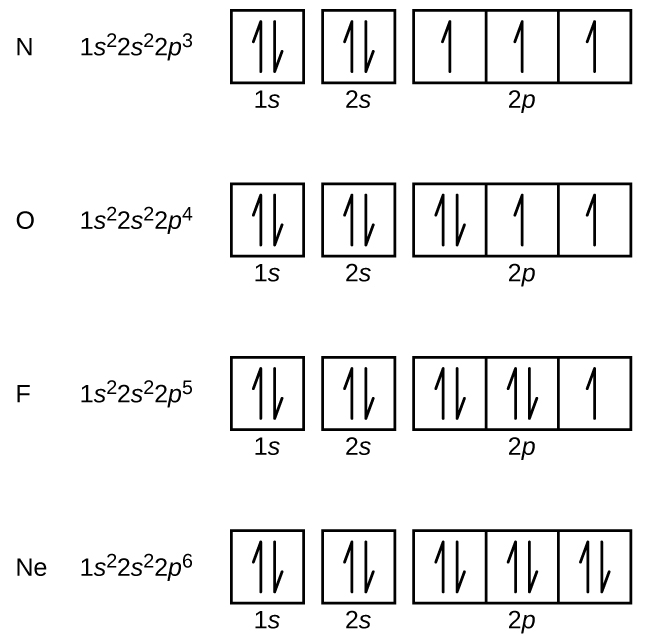
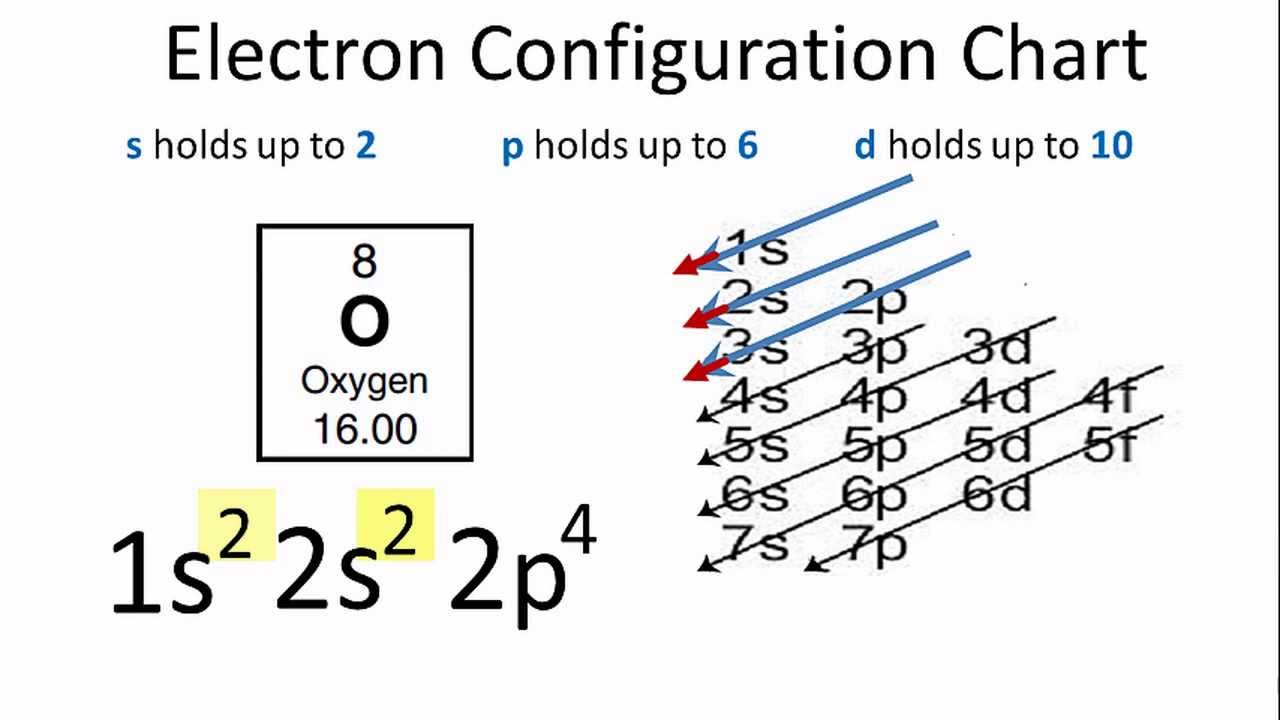
Electron configuration of fluorine (F) atom through orbital diagram Atomic energy levels are subdivided into sub-energy levels. These sub-energy levels are called orbital. The sub energy levels are expressed by 'l'. The value of 'l' is from 0 to (n - 1). The sub-energy levels are known as s, p, d, f.
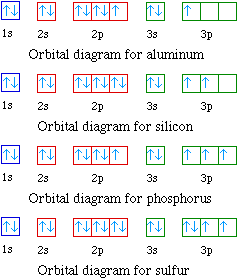
Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. You jump up a little bit in energy and we get the 2s orbital that make it the 2p sublevel. What is the orbital diagram for chlorine?
What does an orbital diagram tell about a given element? An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally.
The orbital wave function or ϕ is a mathematical function used for representing the coordinates of an electron. The square of the orbital wave function or represents the probability of finding an electron. This wave function also helps us in drawing boundary surface diagrams.
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. Arrows (or half arrows) are used to represent the electrons occupying the orbitals. (The paired electrons are said to have antiparallel spin). What object is represented by the half arrows in an orbital diagram?
What is the orbital diagram? Orbital diagrams are a pictorial description of electrons in an atom. In order to figure out where electrons go in an atom we have to follow 3 main rules. The first one being the Auf Bau Principle, the Auf Bau Principle states that each electron occupies the lowest energy orbital available.
The orbital shown in the diagram has one nodal plane. b. An Isosurface plot is used to understand/visualize the hydrogen atomic orbital better with the set of colored surfaces indicating the constant resulting quantity that gives the information on the specific spatial coordinate, where a Foggy plot shows the general direction of the orbital ...
Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).
What is the orbital diagram for Mo? A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. What is the MO configuration for b2?
What is incorrect about this orbital diagram? * 1s 2s 2p O Both arrows in the filled 2p box should be pointing the same direction O All the arrows should be pointing the same direction. O There is nothing incorrect with this diagram O In the there should only be 1 arrow in the first 2p box and one in the 2nd 2p box. check_circle.

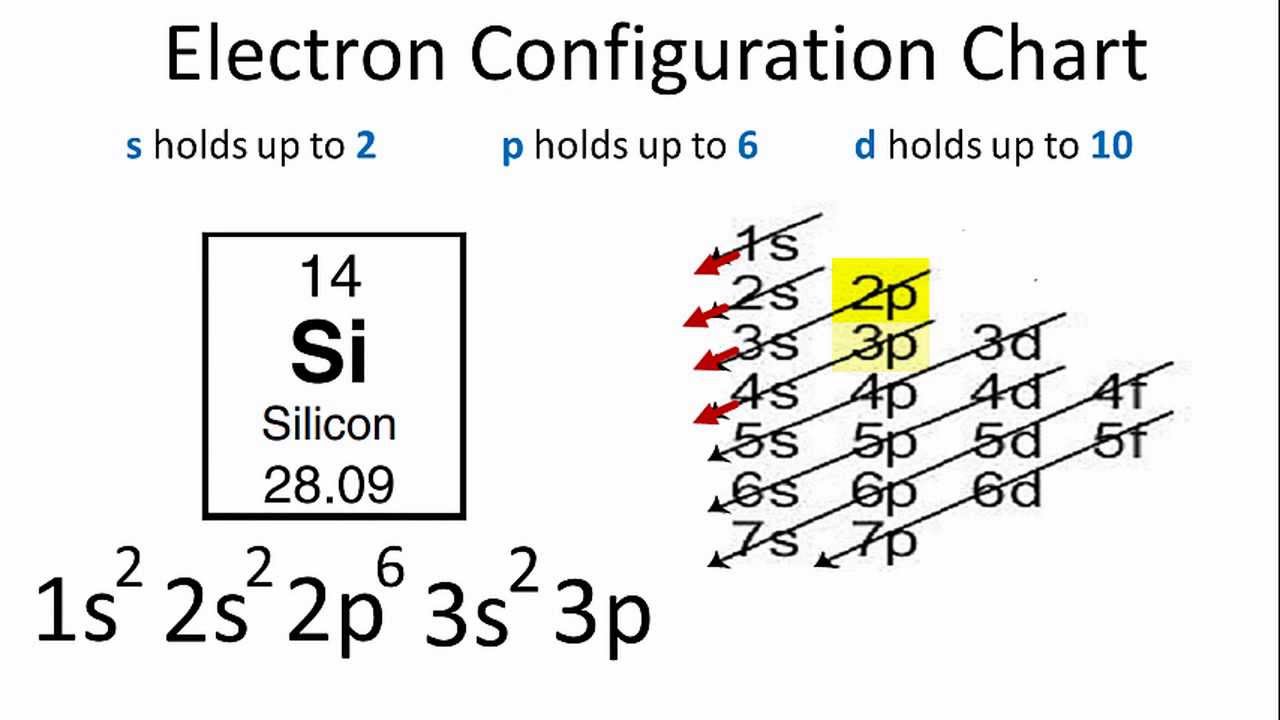
draw a partial valence level orbital diagram and write the condensed ground state electron configu 3





















0 Response to "37 what is orbital diagram"
Post a Comment