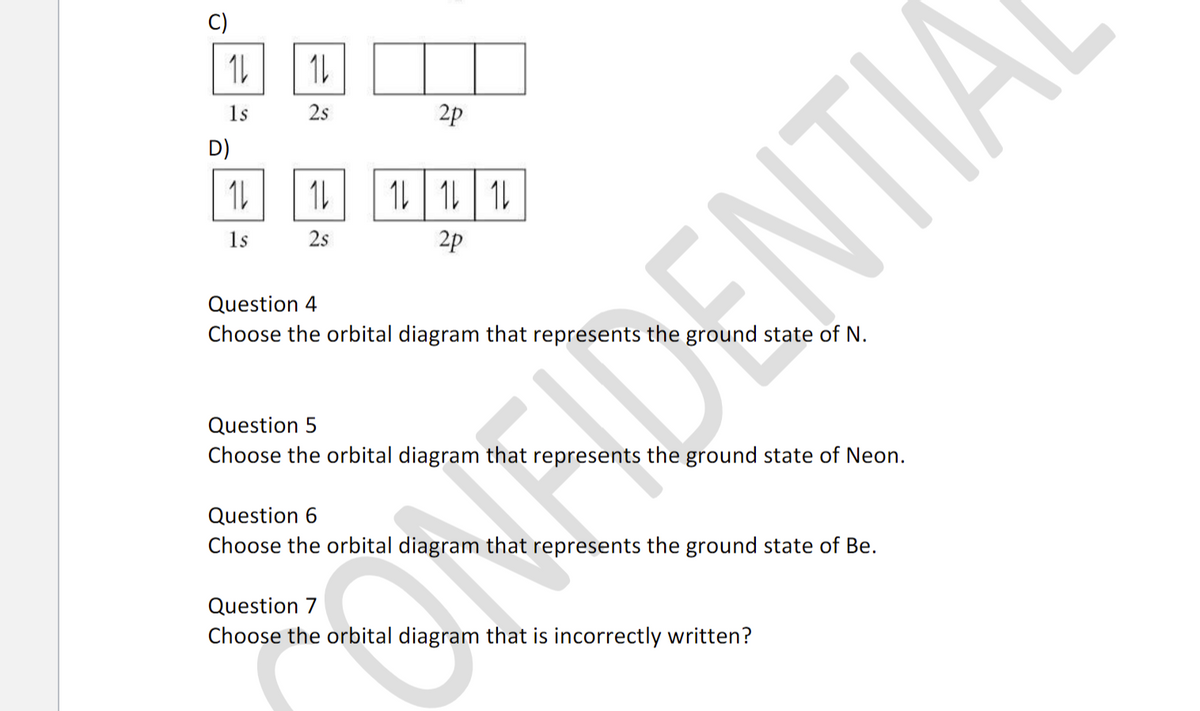
39 orbital diagram for neon
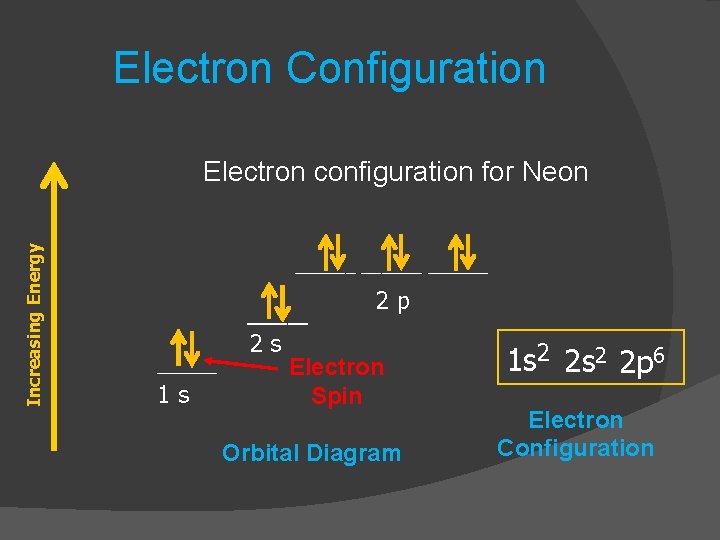
Fill in the orbital energy diagram for the neon atom. 2p El 2s 1s Submit Answer Try Another Version 2 item attempts remaining ; Question: Fill in the orbital energy diagram for the neon atom. 2p El 2s 1s Submit Answer Try Another Version 2 item attempts remaining . This problem has been solved! Orbital diagram. Tags: Question 12. SURVEY. 300 seconds. Q. The electron configuration of an atom is 1s 2 2s 2 2p 6. The number of electrons in the atom is. answer choices.
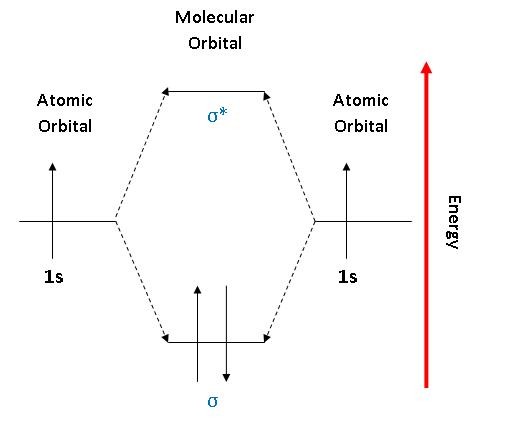
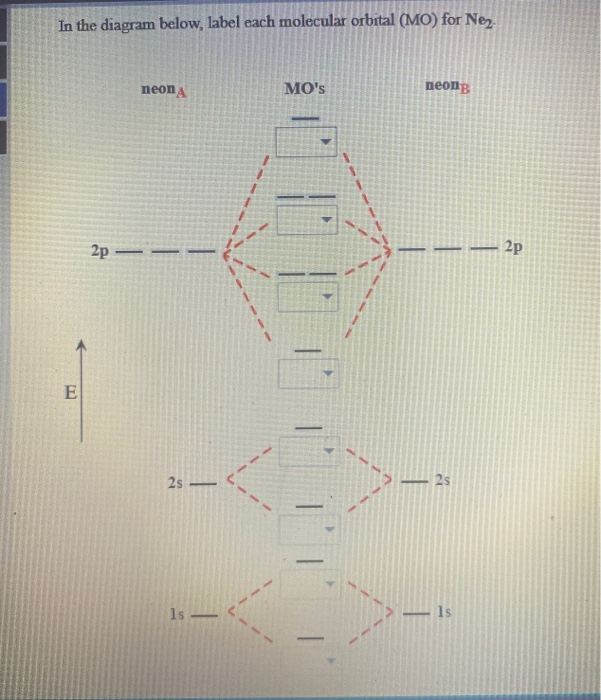
Molecular Orbital Diagram of Neon Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET.Wat...

Orbital diagram for neon
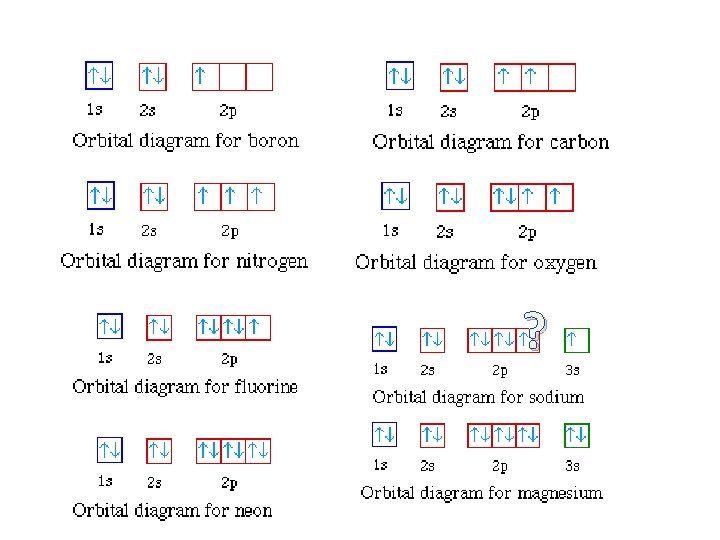
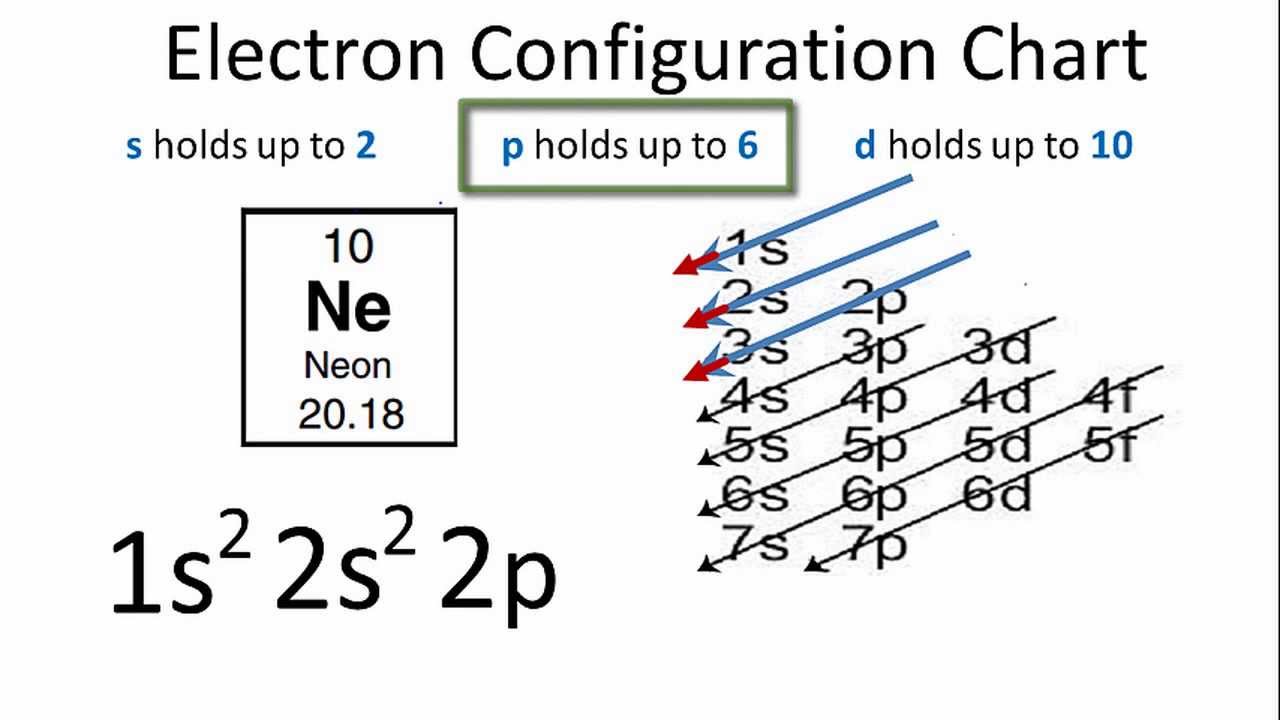
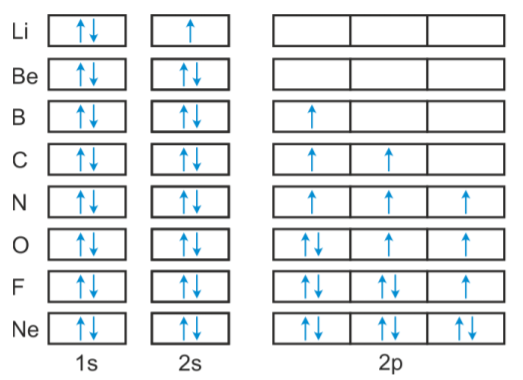
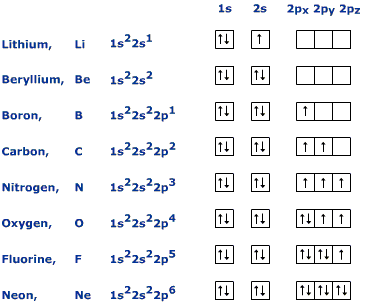
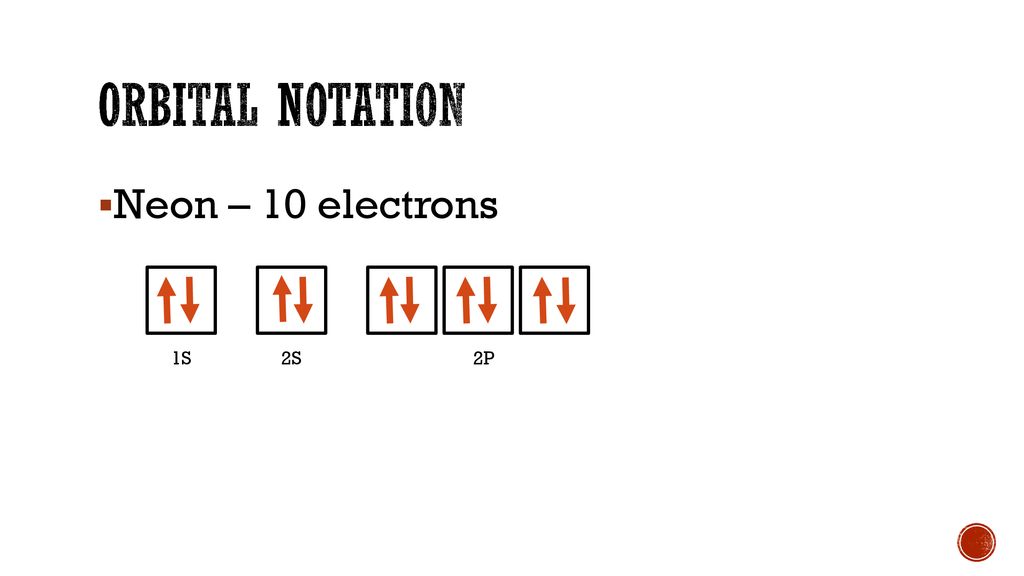
Orbital Diagram for Neon. molecular orbital theory home faculty molecular orbital theory the goal of molecular orbital theory is to describe molecules in a similar way to how we describe atoms that is in aufbau principle indiana university northwest the equations of modern atomic theory are difficult to solve fortunately many of the results can be obatined by following some simple rules Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11 ... Answer and Explanation: 1. For neon, the atomic number is 10. The electronic configuration for neon is 1s22s22p6 1 s 2 2 s 2 2 p 6 . The full orbital diagram for neon is shown below. Orbital diagram.
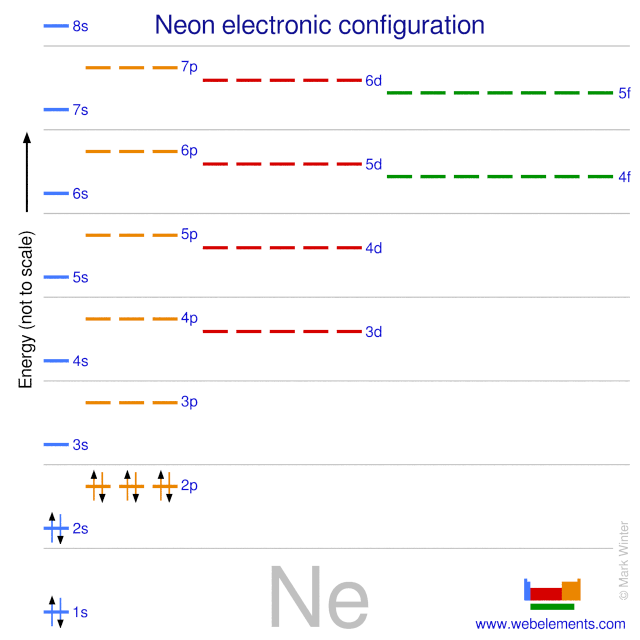
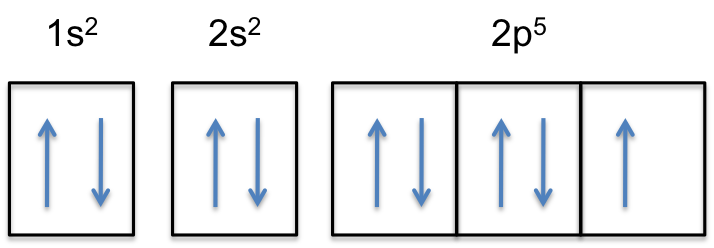
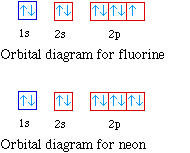
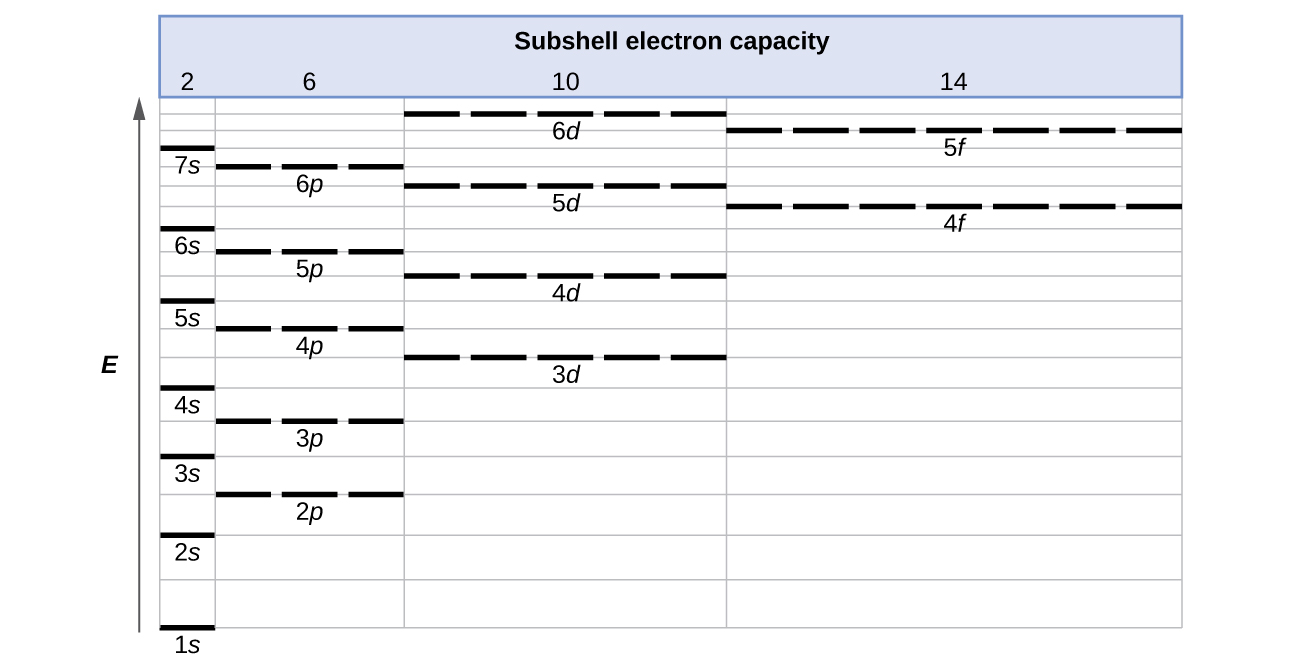
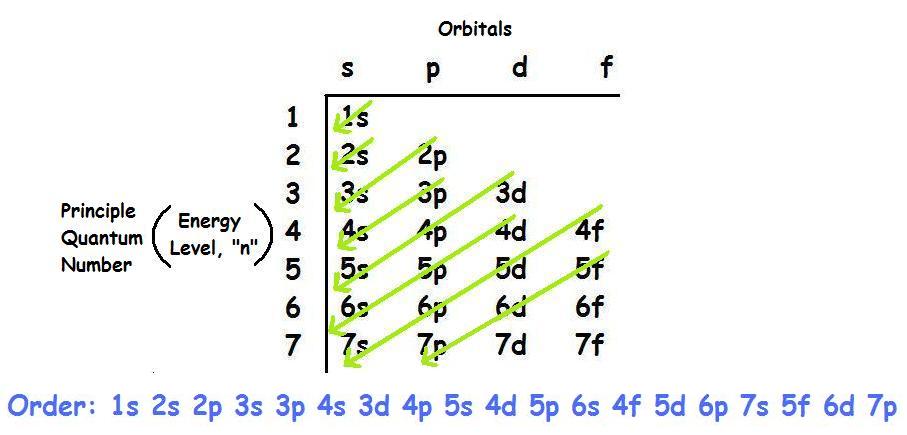
Orbital diagram for neon. Electronic configuration of the Neon atom. Valence electrons. Orbital diagram. Neon electron configuration ← Electronic configurations of elements . Ne (Neon) is an element with position number 10 in the periodic table. Located in the II period. Melting point: -248.7 ℃. Neon electron configuration is 1s 2 2s 2 2p 6.The symbol for neon is ‘Ne’ and it is an inert element. This article gives an idea about the electron configuration of neon(Ne) and orbital diagram, period and groups, valency and valence electrons of neon, application of different principles. The tenth element in the periodic table is neon. The orbital diagrams for fluorine and neon are shown. The next two electrons continue to pair those electrons that are unpaired to fill up the 2p orbitals. With neon the second level is filled with electrons. Completed levels are a characteristic of all noble gases. If we look at the energy level diagram for neon the completed second level ... The electron configuration of neon is: 1s22s22p5. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with ...
In writing the electron configuration for neon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Ne go in the 2s orbital. The remaining six electrons will go in the 2p orbital. Therefore the Ne electron configuration will be 1s 2 2s 2 2p 6. Because the second energy level (2s 2 2p 6) has eight electrons Neon has an octet and has a full outer shell. Answer and Explanation: 1. For neon, the atomic number is 10. The electronic configuration for neon is 1s22s22p6 1 s 2 2 s 2 2 p 6 . The full orbital diagram for neon is shown below. Orbital diagram. Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11 ... Orbital Diagram for Neon. molecular orbital theory home faculty molecular orbital theory the goal of molecular orbital theory is to describe molecules in a similar way to how we describe atoms that is in aufbau principle indiana university northwest the equations of modern atomic theory are difficult to solve fortunately many of the results can be obatined by following some simple rules





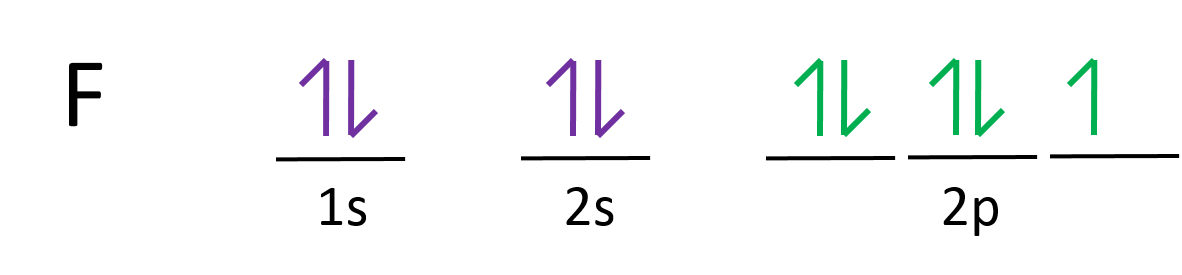
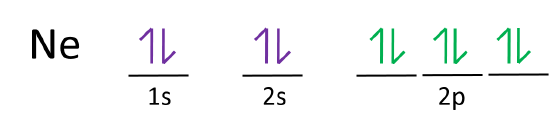





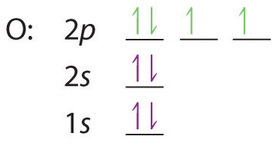




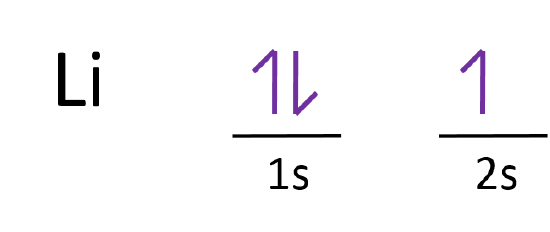
0 Response to "39 orbital diagram for neon"
Post a Comment