40 orbital diagram worksheet with answers
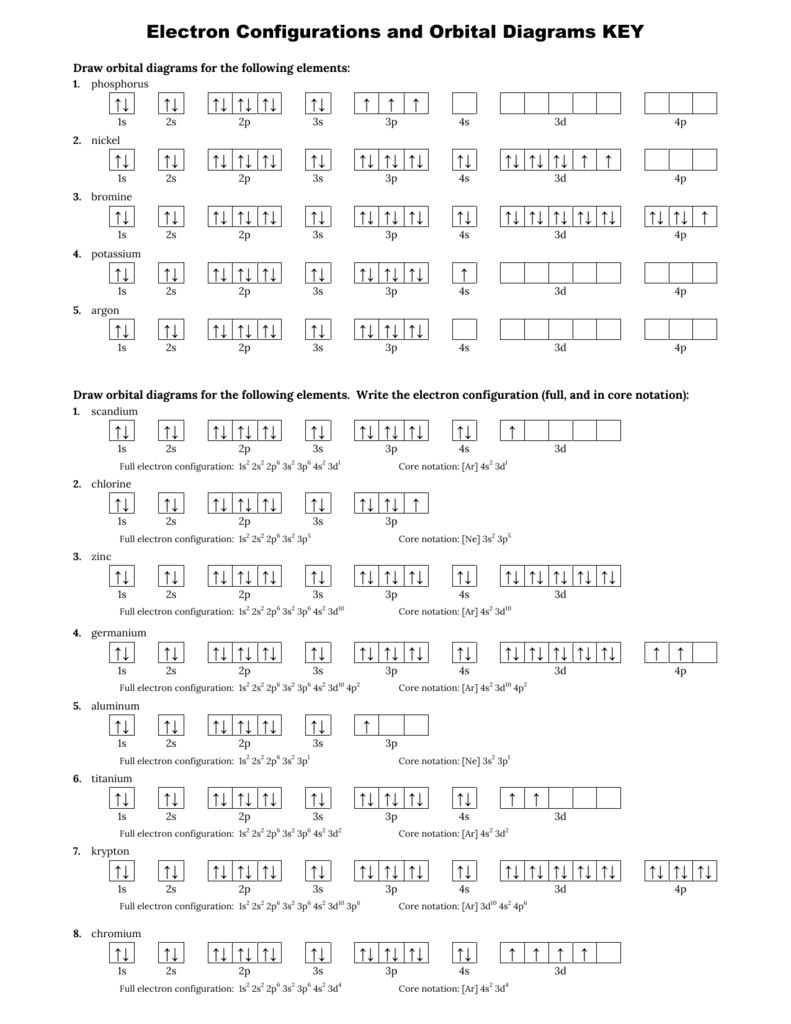
Orbital diagram worksheet with answers. THE AUFBAU PRINCIPLE. Fe, Ni and Zn atoms. 6. Two electrons in the same orbital must have opposite spin so the arrows are drawn pointing in opposite directions. Worksheet The Nature Of Science Worksheet Answers chapter 2 1 review. ) You will need the Oh character table. The first electron in on orbital is represented by a and the second by A Set of … Use the orbital filling diagrams to complete the table. Is 2s lectron Is 4s on 2s a o o gurations or ome Orbital filling elected ements Electron 3s configuration Isl C] element (answer) en on Element O Ne 2Px 2py 2pz 2. Which element has the following orbital diagram? 3. Using arrows, show how the following orbitals will fill with electrons.
An awesome collection of free atomic structure worksheets for teachers. An atom is the smallest constituent unit of ordinary matter. It is composed of protons, neutrons, and electrons. Get more information about atoms on our website.

Orbital diagram worksheet with answers
proportional). This throws out answers (A) and (B). If the velocity was to be half of what it was before (Ans. E), the orbital distance would have to quadruple (since you take the square root of the distance between the objects). This leaves only answers (C) and (D). Compare then 𝑣𝑜=√ 𝐺∙𝑀 6400 and 𝑣=√𝐺∙𝑀 6800 The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2s and then 2p, 3s, and 3p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. However, this pattern does not hold for larger atoms. The 3d orbital is higher in energy than the 4s orbital. Such ... 1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following ...
Orbital diagram worksheet with answers. Start with a diagram. You could draw a top view of this cyclist like I did, but it isn't necessary. Do draw an arrow pointed to the right, however, to represent the direction of the cyclist. Wind directions are measured clockwise from due north. North is 0°, east is 90°, south is 180°, and west is 270°. The wind is coming from 248°, which lies somewhere between south and west. Draw an ... 11.12.2012 · Circular Motion Problems – ANSWERS 1. An 8.0 g cork is swung in a horizontal circle with a radius of 35 cm. It makes 30 revolutions in 12 seconds. What is the tension in the string? (Assume the string is nearly horizontal) T=time/revolutions=0.4 s Period is the time per revolution F=ma Write down N2L F tension = mv 2/r Tension provides net force, acceleration is centripetal F tension =m(4 2r ... An orbital defines a region within an energy level where there is a high probability of finding a pair of electrons. There can be a maximum of two electrons in each orbital. This is why the electrons are often shown in pairs within an energy level. Tell students that the rows across on the periodic table are called periods. Period 1 Hydrogen The following molecular orbital diagram may be used for the following problems. For oxygen and fluorine, the σ 2p orbital should be lower in energy than the π 2p. However, the diagram will still yield correct bond order and magnetic behavior for these molecules.
1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following ... The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2s and then 2p, 3s, and 3p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. However, this pattern does not hold for larger atoms. The 3d orbital is higher in energy than the 4s orbital. Such ... proportional). This throws out answers (A) and (B). If the velocity was to be half of what it was before (Ans. E), the orbital distance would have to quadruple (since you take the square root of the distance between the objects). This leaves only answers (C) and (D). Compare then 𝑣𝑜=√ 𝐺∙𝑀 6400 and 𝑣=√𝐺∙𝑀 6800

Worksheet Orbital Diagrams Teacher Teacher Notes Name Key Class Date Orbital Diagrams Electron Configuration 1 Compare And Contrast Hunds Rule Course Hero
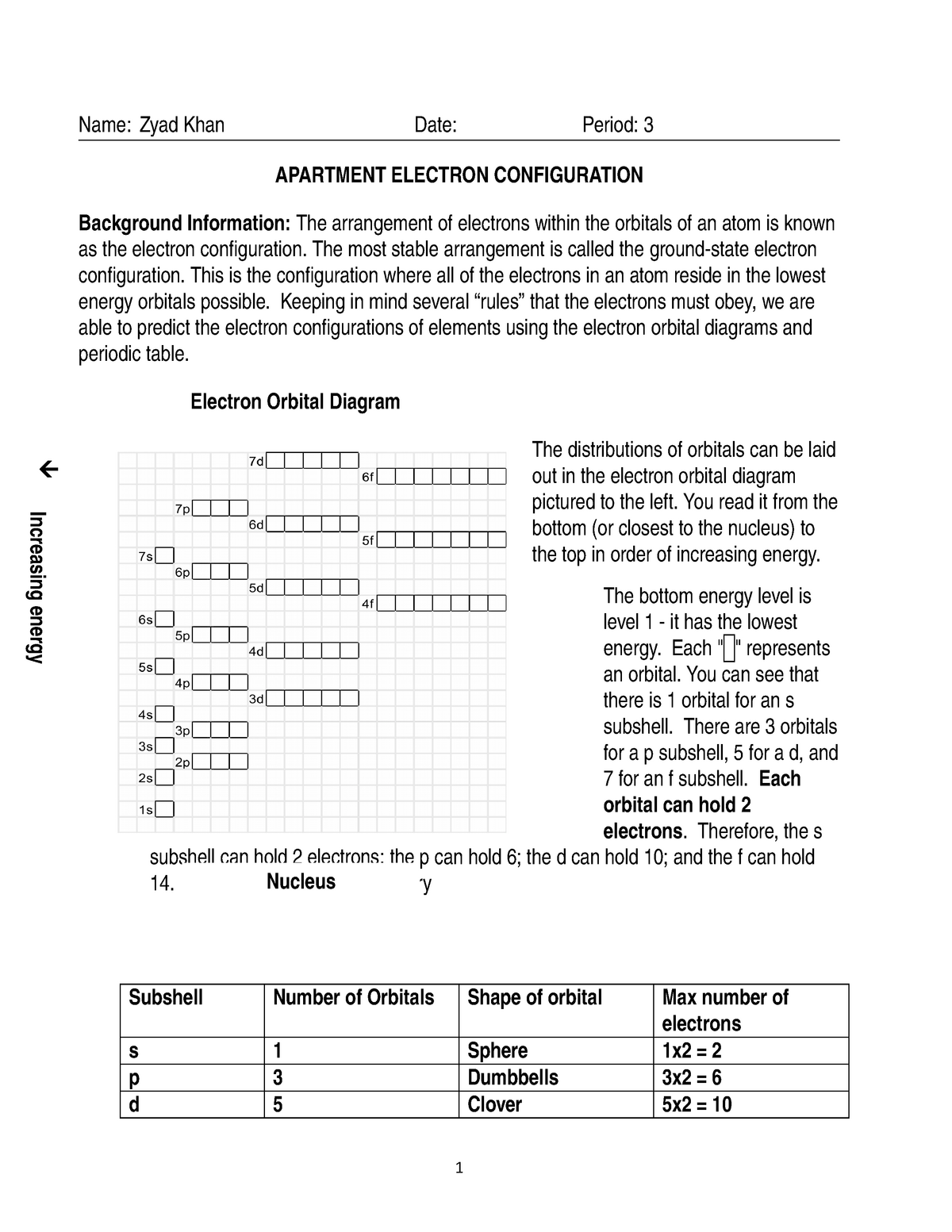
Electron Configuration Activity 2 Name Zyad Khan Date Period 3 Apartment Electron Configuration Studocu
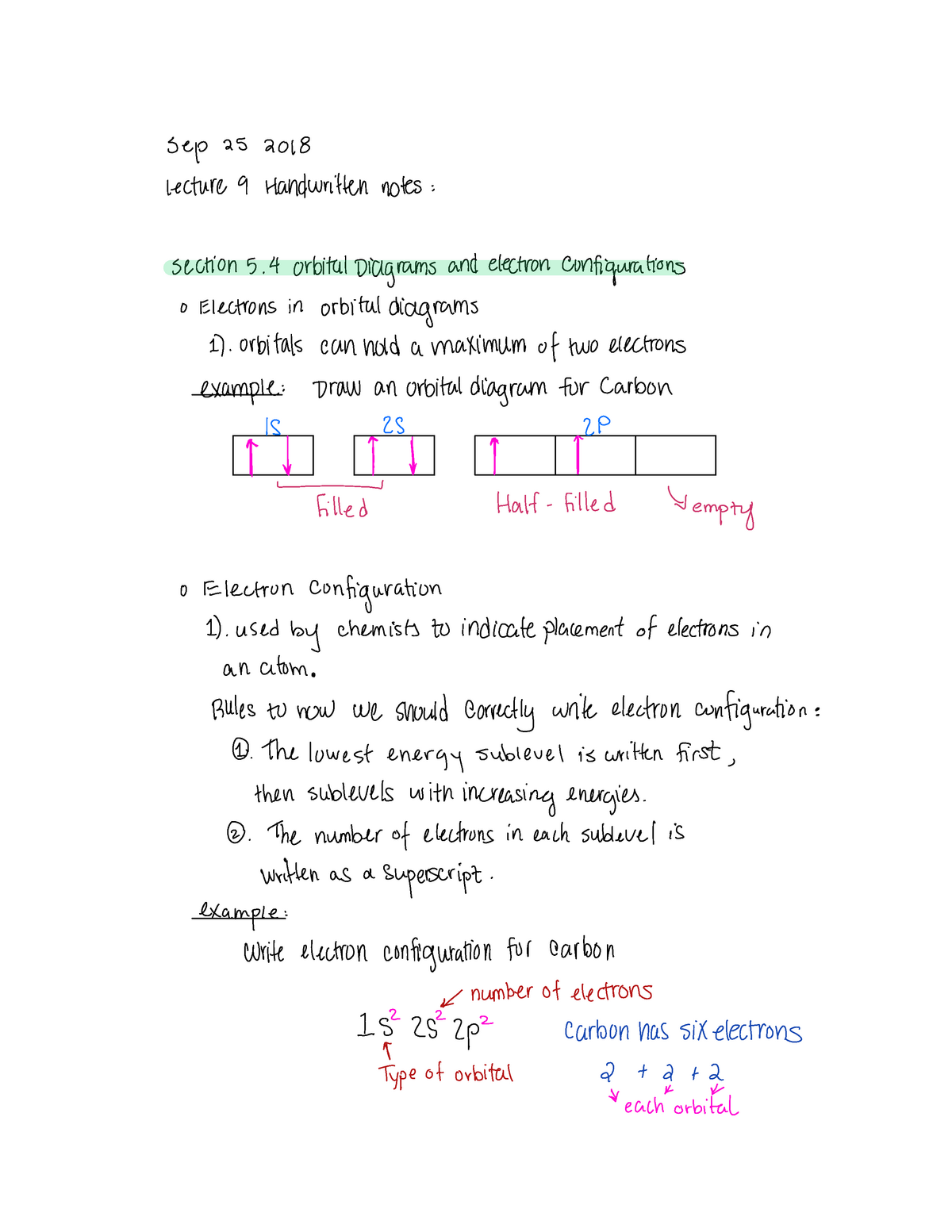
Orbital Diagrams And Electron Configurations Sep 25 2018 Lecture 9 Handwritten Notes Section 5 4 Studocu

Kami Export Ryan Smith Orbital Diagram Worksheet Pdf Protons Which Is Known As The Atomic Number Next The Boxes Are Drawn For The Orbitals Arrows Course Hero

Kami Export Kivanc Yavuz Oca 5 Orbital Diagrams Worksheet Pdf Orbital Diagrams Chem Worksheet An Orbital Diagram Uses Name Boxes With Arrows To Course Hero

Orbital Diagram Pdf 2s 1s 2s 1s 2s 1s 2p 2p 2p 2p 2p 2p Printable Worksheets Www Mathworksheets4kids Com 2s 1s 6 Copper 5 Iron 2s 1s 4 Silicon 3 Course Hero






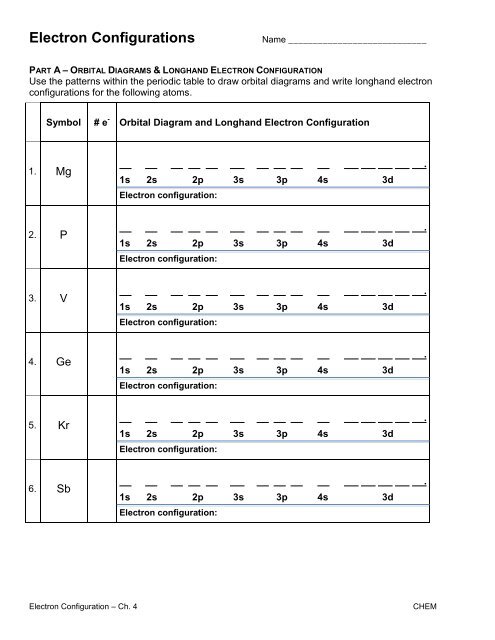




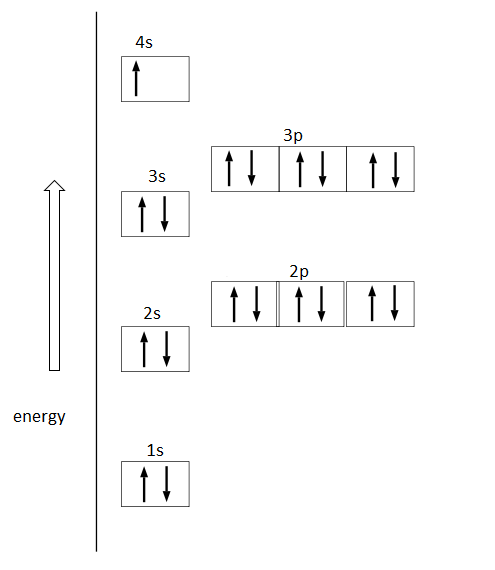














0 Response to "40 orbital diagram worksheet with answers"
Post a Comment