40 lewis dot diagram of ammonia
August 1, 2016 - Answer (1 of 3): One thing you know right off the bat is that nitrogen is in the middle. Hydrogen only has one valence electron, so it will just have the one bond. Draw N in the middle and draw the “H”s around it, one on each side and one either on the top or the bottom (it doesn’t matter ... Are you looking for the best Science-education resources on the internet? Look no further, you've found them!
January 1, 2019 - Ammonia is related to ammonium (NH4+), whose lewis structure is shown here. Ammonia is generally a base. It is a type of nitrogenous waste especially prevalent in aquatic organisms. It is considered a hazard toward human health and can have the effects on the neural system as shown in the diagram ...

Lewis dot diagram of ammonia
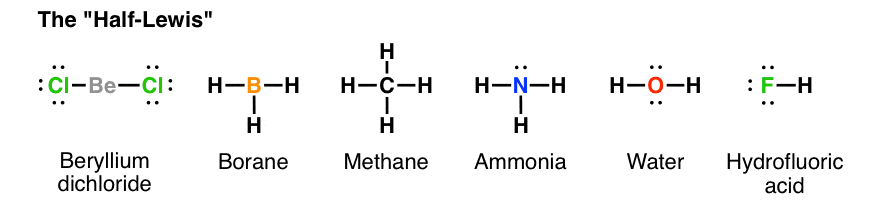
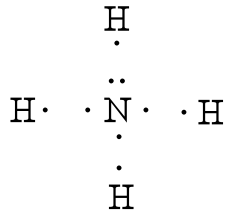
Draw an electron dot diagram to show the formation of ammonium ionAtomic number N7 and H1 September 17, 2020 - Nh3 Lewis Structure has the The formula of ammonia is NH3. it’s liquid state molecules ... The Lewis Structure or the Lewis Dot Diagram or the Lewis Dot Structure, named after Gilbert N. Lewis, shows the pictorial representation of the atomic bonding of the molecules or an element. Group 15 elements such as nitrogen have five valence electrons in the atomic Lewis symbol: one lone pair and three unpaired electrons. To obtain an octet, these atoms form three covalent bonds, as in NH3 (ammonia). Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds:
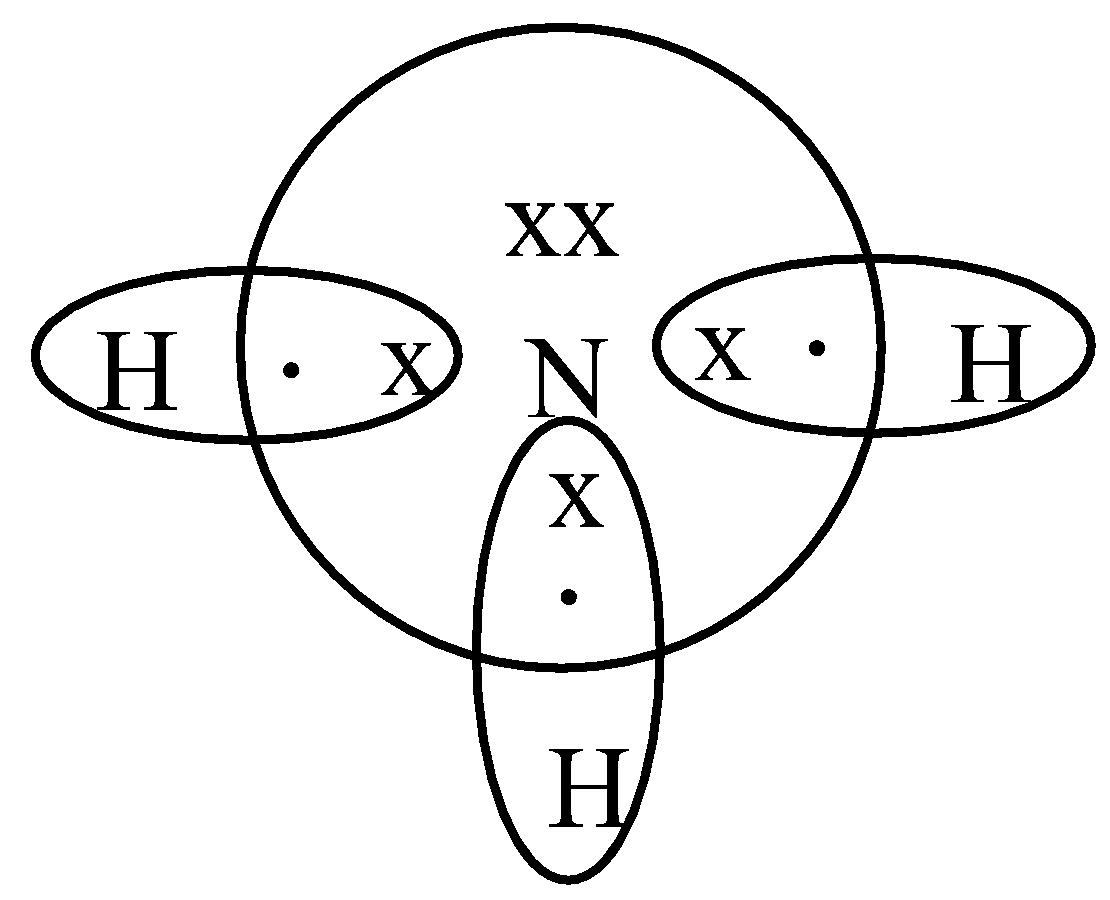
Lewis dot diagram of ammonia. Lewis Structures for NH3. Step-by-step tutorial for drawing the Lewis Structure for Ammonia. A step-by-step explanation of how to write the Lewis Dot Structure for NH3 (Ammonia or Nitrogen Trihydride). The Lewis structure for NH3 is one of the most c... January 10, 2019 - We show two ways to draw the NH3 Lewis structure, ammonia. We also have a handy video on the 5 things you need to know for general chemistry December 10, 2016 - There are 8 valence electrons to distribute........... And 3xxN-H bonds, and one nitrogen-centred lone pair of electrons.
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation Ammonia lewis structure contains three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH3 can be drawn by using valence electrons of nitrogen and hydrogen atoms. Steps of drawing the lewis structure of NH3 is explained in detail in this tutorial. February 7, 2017 - Answer: You look up “electron dot diagram for ammonia” on google images. It really isn’t that hard. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, 2012 - Lewis Electron Dot Structure for ammonia molecule NH3
July 23, 2021 - Ammonia is a colorless compound, used in making fertilizers. It is a stable hydride formed of one nitrogen and three hydrogen atoms. The molecule has a pungent smell. It can form an NH4+ ion by accepting a proton. In this blog post, we will learn about the Lewis dot structure, electron geometry, ... By looking at the Lewis dot structure of ammonia NH3 molecule the shape of the ammonia molecule is described by which one of the following aTrigonal pyramidal bTshaped cSquare planar dTrigonal planar November 30, 2012 - Video by Janet Gray Coonce MS If you plan to view the video on your cell phone, consider your data plan and whether you should wait until you have a WiFi connection to avoid cellular charges. A Wi-fi connection will prevent cellular data charges for video streaming. December 6, 2020 - In addition to this, ammonia is considered corrosive as well as hazardous if stored in significantly larger quantities. The lewis structure that is also called an electron dot structure, is mainly a pictorial representation of the valence electrons present in an atom. The diagram is drawn using ...
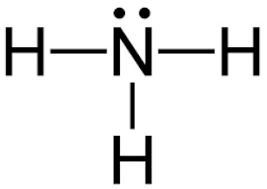
May 5, 2018 - Have a look here... The Lewis structure of ammonia, NH_3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons.
From Wikipedia, the free encyclopedia Jump to navigationJump to search This article is about the cat species that is commonly kept as a pet. For the cat family, see Felidae. For other uses, see Cat (disambiguation) and Cats (disambiguation). For technical reasons, "Cat #1" redirects here. For the album, see Cat 1 (album). Domestic cat[1] Cat poster 1.jpg Various types of domestic cat Conservation status Domesticated Scientific classification e Kingdom: Animalia Phylum: Chordata Class: Mammalia O...
Lewis Dot of Ammonia · Back70 More Lewis Dot StructuresSince all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule
Group 15 elements such as nitrogen have five valence electrons in the atomic Lewis symbol: one lone pair and three unpaired electrons. To obtain an octet, these atoms form three covalent bonds, as in NH3 (ammonia). Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds:
September 17, 2020 - Nh3 Lewis Structure has the The formula of ammonia is NH3. it’s liquid state molecules ... The Lewis Structure or the Lewis Dot Diagram or the Lewis Dot Structure, named after Gilbert N. Lewis, shows the pictorial representation of the atomic bonding of the molecules or an element.
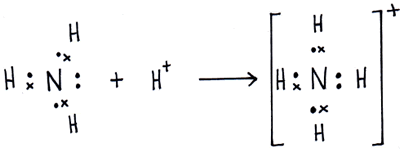
Draw an electron dot diagram to show the formation of ammonium ionAtomic number N7 and H1
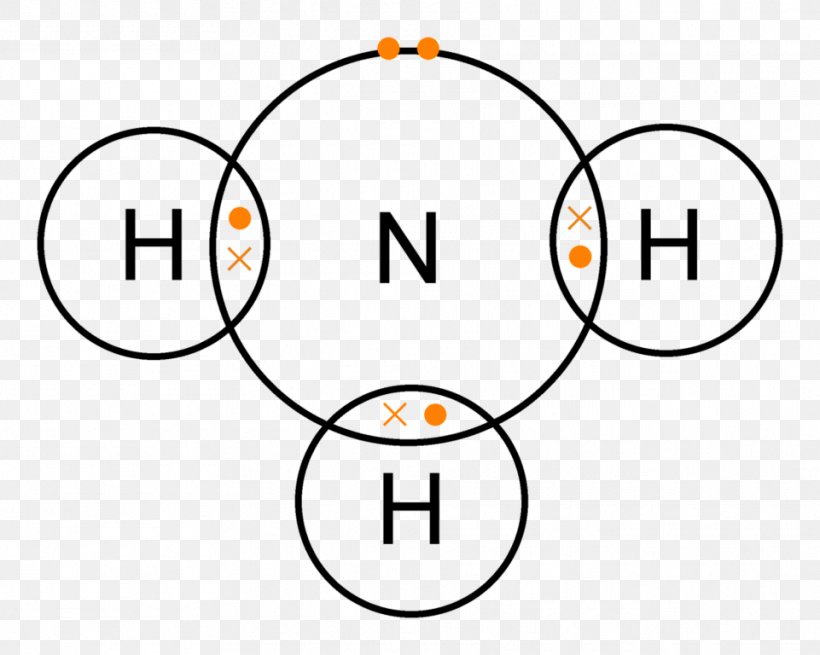
Lewis Structure Ammonia Covalent Bond Lone Pair Chemical Bond Png 961x768px Lewis Structure Ammonia Area Atom

Draw Electron Dot Structure For Ammonium Nh4 Science Carbon And Its Compounds 11147571 Meritnation Com

Lewis Structure Ammonia Lone Pair Ammonium Lewis Pair Mason Jar Model Prototype Angle Text Png Pngegg

Give The Lewis Structures For The Following Compounds Include All Bonding Pairs And Non Bonding Lone Pairs A Nh3 B H2s C Co2 D Sih4 E C2h6 Study Com
What Is The Valency Of Nitrogen In Ammonia Draw Its Electron Dot Structure Also Chemistry Topperlearning Com Zdskl5z44
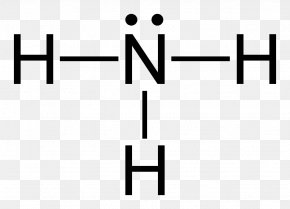
Lewis Structure Ammonia Molecule Chemistry Ammonium Png 640x462px Watercolor Cartoon Flower Frame Heart Download Free

Lewis Dot Notation And Molecular Structure Infographic Diagram With Examples Of Water Ammonia Methane Sulfur Trioxide And Dinitrogen Pentoxide Molecules For Chemistry Science Education Royalty Free Cliparts Vectors And Stock Illustration Image

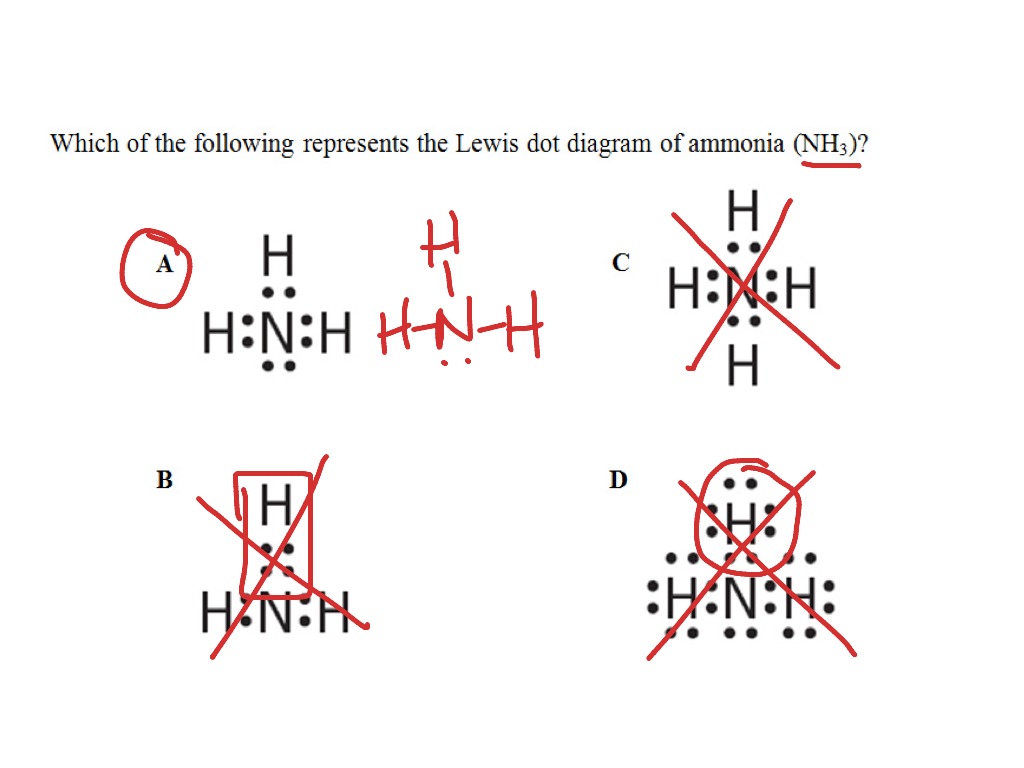
Which Of The Following Is The Correct Lewis Structure For Ammonia Nh3 Note Hydrogen And Nitrogen Brainly Com

Best Answer Draw The Electron Dot Structure Of Ammonia Molecule And Show The Formation Of Ammonium Brainly In




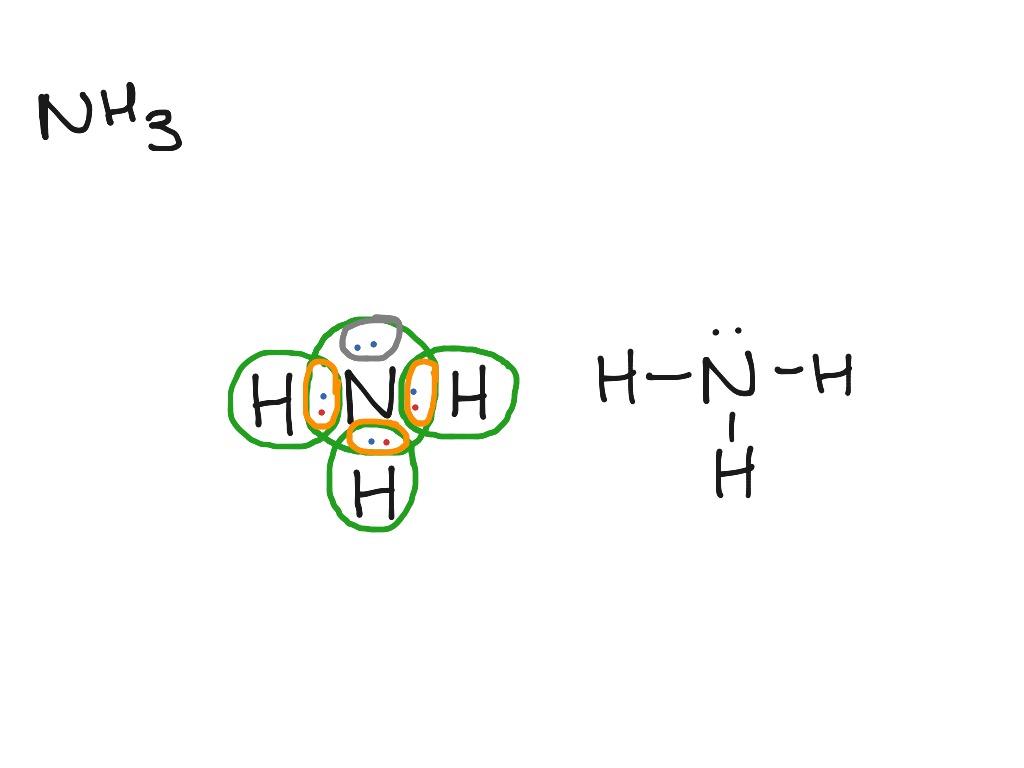

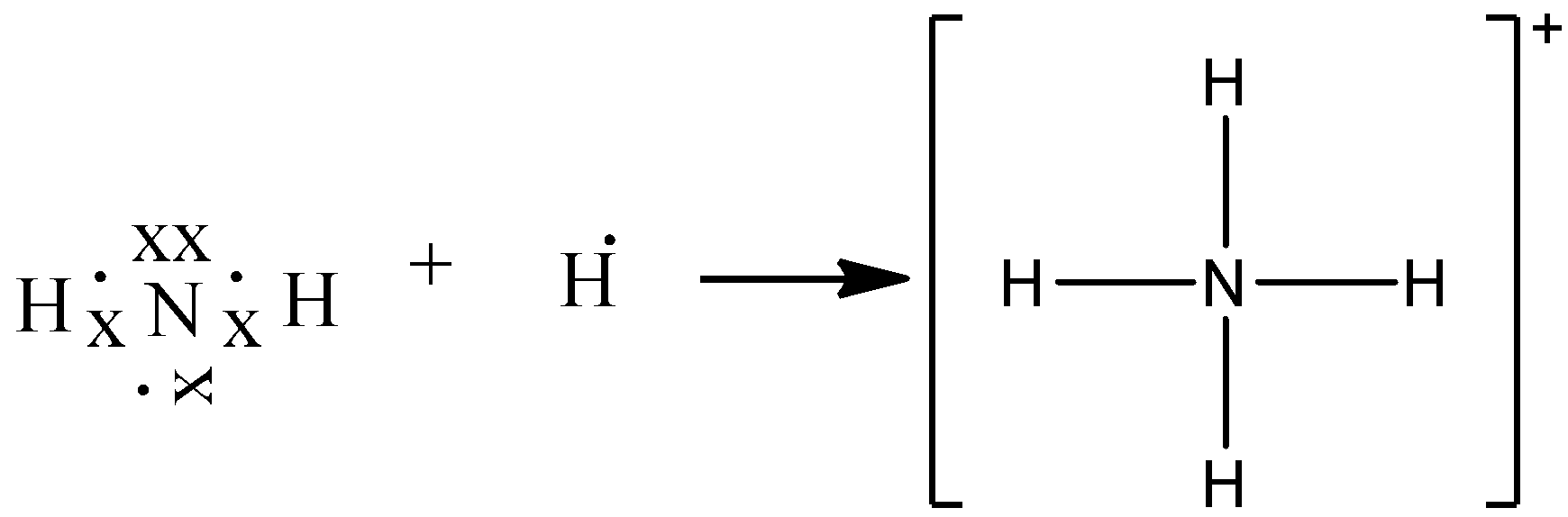









0 Response to "40 lewis dot diagram of ammonia"
Post a Comment