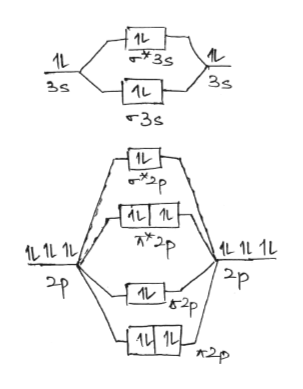
38 mg2 molecular orbital diagram
Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The result is a slight change in the relative energies of the molecular orbitals, to give the diagram shown in the figure below. 0 Comments. on Orbital Diagram For Germanium. orbital. Because an electron can have either one of two spins, any orbital can hold a maximum of four . The orbital diagram for germanium is. 1s. 2s. 2p. 3s. Oxidation States, +4,2. Electrons Per Shell, 2 8 18 4. Electron Configuration, [Ar] 3d10 4s2 4p2. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2.
#"O"_2# is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that #"CO"# is not (as it has zero unpaired electrons), but #"NO"# is (it has one unpaired electron). Well, the MO diagram for #"O"_2# is: The bond order is already calculated in the diagram.

Mg2 molecular orbital diagram
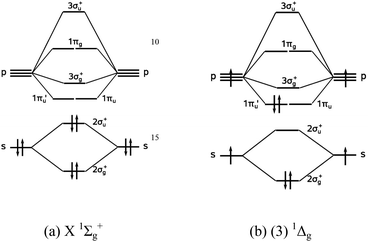
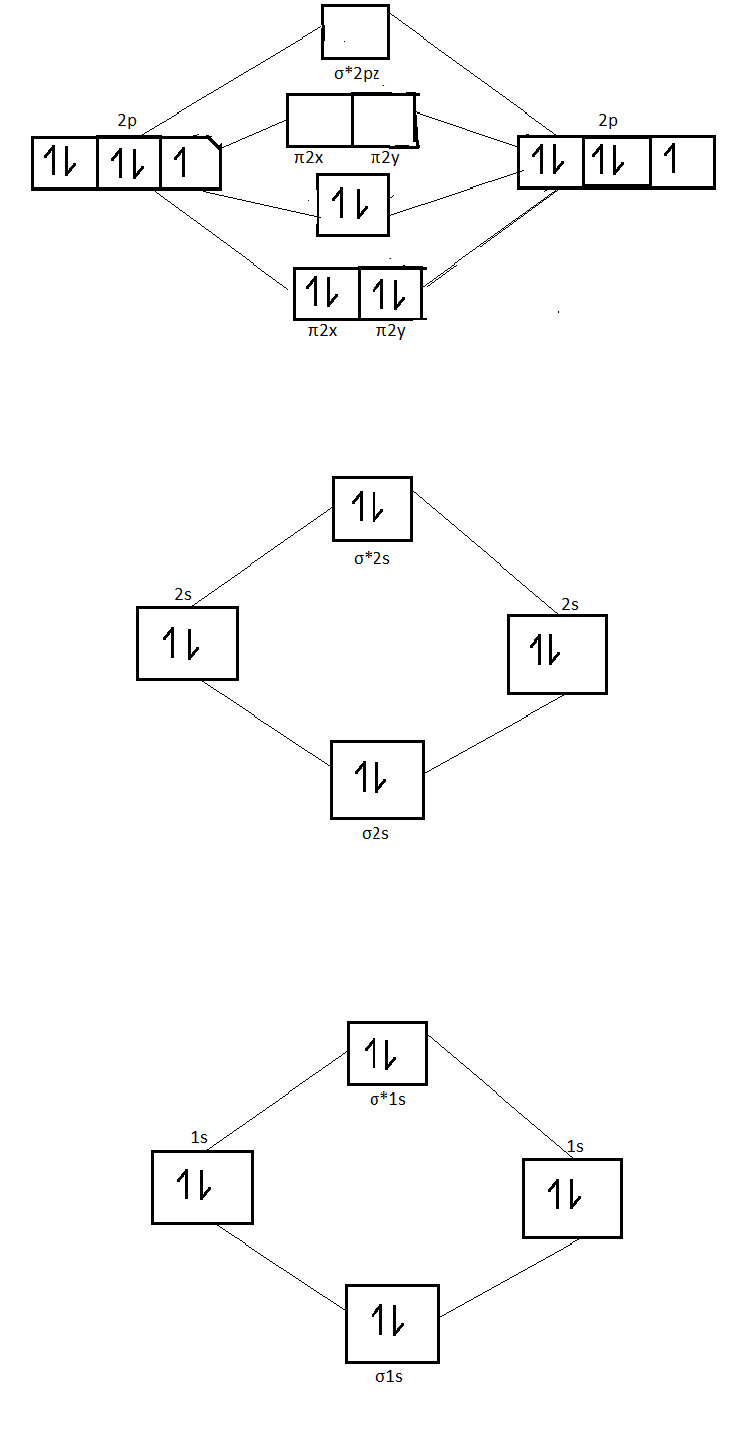
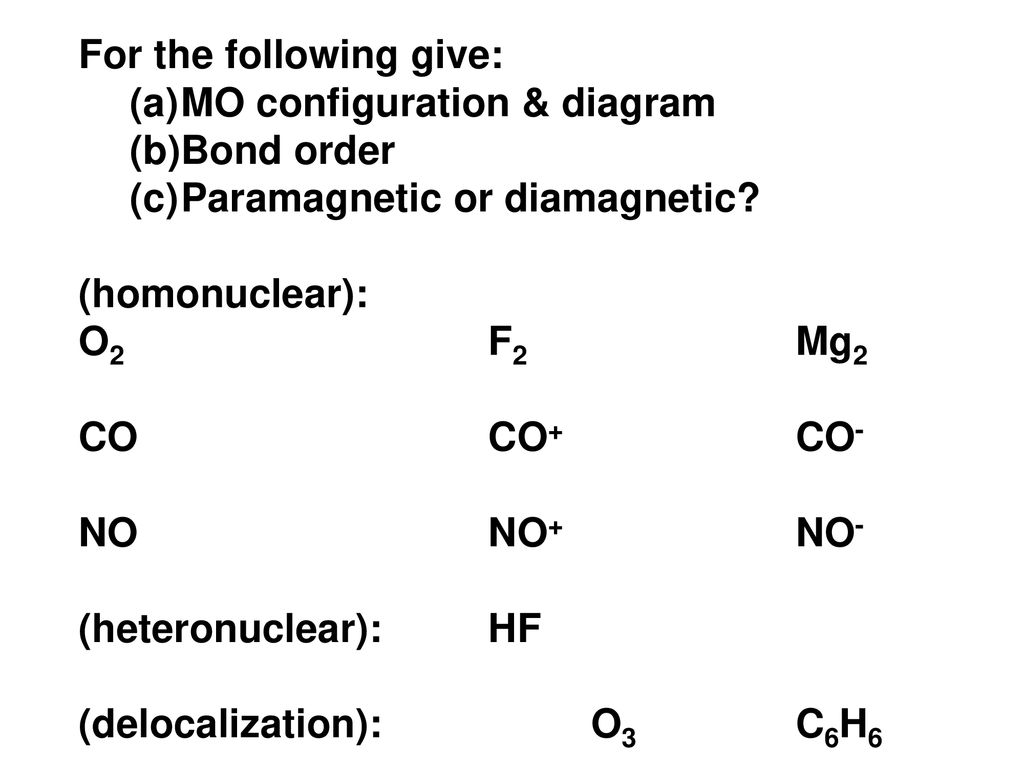
Potential curves of the A 1 Σ u+ - X 1 Σ g+ system of the Mg 2 molecule for J = 0 and J = 71. Reference. Figure 3. Potential curve for Mg 2 calculated within a basis of 56 contracted Gaussian type orbitals (the calculation of the correlation energy used 54 molecular orbitals since the 1σ g and 1σ u orbitals were frozen). Reference. Place the following atoms/ions (Na+, Na, Mg2+) in order by increasing size. Mg2+ < Na+ < Na. What is the standard enthalpy of formation for CaCl2(s)? (Question 8 M3V3) ... Consider the following molecular orbital diagram for O2. Which molecular orbital below corresponds to the molecular orbital labeled with the number 3? (question 30 M3V3) III. Get an easy, free answer to your question in Top Homework Answers. Predict whether or not a Mg2 molecule is stable. a. Mg2 is stable with a bond order of 2b. Mg2 is stable with a bond order of 0.5c. Mg2 is stable with a bond order of 1d. Mg2 is not stable Get an easy, free answer to your question in Top Homework Answers.
Mg2 molecular orbital diagram. At http://ecampus.oregonstate.edu/chemistry, you can earn college credit for online Chemistry and virtual labs. With no onsite visits required for 100 level ... the pi(2p) bonding orbitals are LOWER than the sigma(2p) bonding orbitals.N2(2-) has a bonding order of 2, which predicts that there will be a stable double ... This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic orbitals. Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ... Molecular Orbital Diagram Maker. These quizzes enable you to build your own molecular orbital diagram from components. A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. 13. Draw the molecular orbital diagram for the molecule Mg2. Determine the bond order of the molecule and indicate whether or not the molecule exists. Question: 13. Draw the molecular orbital diagram for the molecule Mg2. Determine the bond order of the molecule and indicate whether or not the molecule exists. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.Electron Configuration for Beryllium (Be)Hybrid orbitals - 1 ...
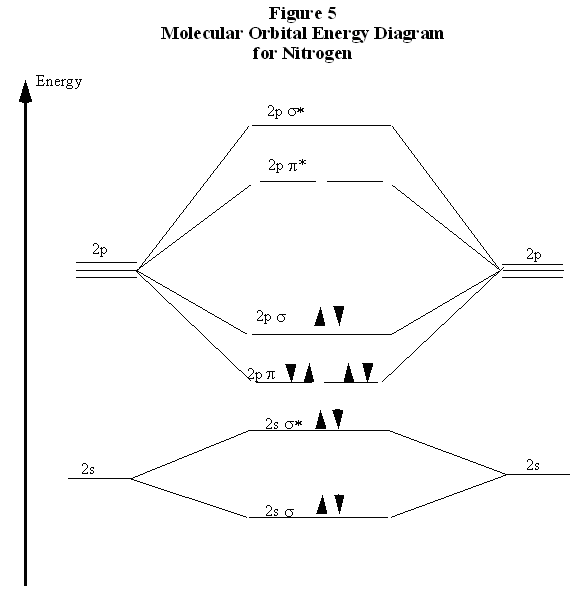
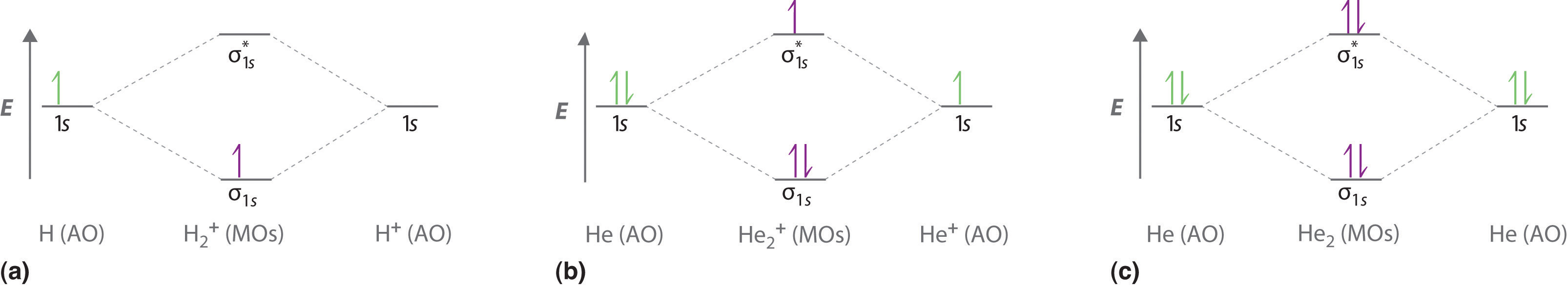
Identify the ground-state electron configuration for Mg2⁺. A) 1s22s22p63s1 B) 1s22s22p63s2 C) 1s22s22p6 D) 1s22s22p63s23p2 E) 1s22s22p63s23p6 ... When two atomic orbitals come together to form two molecular orbitals, one molecular orbital will be lower in energy than the two separate atomic orbitals and one molecular orbital will be higher in ... Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron. If we build the MO diagram for "N"_2, it looks like this: First though, notice that the p orbitals are supposed to be degenerate. They weren't drawn that way on this diagram, but they should be. Anyways, for the electron configurations, you would use a notation like the above. g means "gerade", or even symmetry upon inversion, and u means "ungerade", or odd symmetry upon inversion. Answer: This just shows energy levels so let's take this a step further. Atomic Electron Configurations And I'm not having any luck but if you go to this site, you should be about to see what the 1s, 2s, 2px, 2py, 2pz, and 3s orbitals look like together. Jmol orbital structures If not, see what...
etc.) and each molecular orbital (s2s, p2p. x, p2p. y, etc.) that you draw. • Bond order = ½(6-4) = 1 . Fill in the electrons for both the atomic and molecular orbitals. 2. (a) Write the valence electron configuration (from lowest to highest orbital energies) for the ion N-1 2. Your answer should be in a form similar to (s. 2s) 2, which is ...
Bond Order: Bond order is the number of bonds, or shared electron pairs, between atoms, which can be calculated by using this equation: 0.5 x (number of bonding electrons - number of non-bonding ...
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
A molecular orbital diagram, Beryllium has an electron configuration 1s 2 2s 2, so there are again two electrons in the valence level. However, the 2s can mix with the 2p orbitals in diberyllium, whereas there are no p orbitals in the valence level of hydrogen or helium. The orbital filling diagram of boron. I skipped past beryllium because I ...
The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 8.34). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons.
Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ...
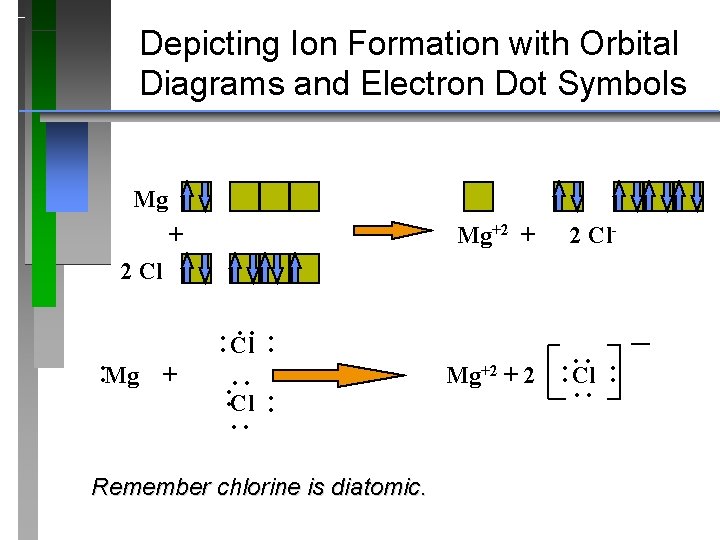
Mg2+ is a Mg atom that now has the same number of electrons as a noble gas. It achiveved this by giving up to other atoms in its vicinity who could be considered "electron acceptors": they are atoms from chemical elements that are ready to accept these electrons to reach the configuration of a noble gas.
What is an bond electron transfer how electron dot diagram for s awesome sulfur atom flow block orbital diagram for magnesium awesome lewis electron dot diagram s. Magnesium reacts with sulfur to produce sulfide a in. That is, magnesium is a cat ion element. Magnesium donates the electron of the last shell to for m bonds and turns into ...

For The Molecule He2 A Construct The Mo Diagram Assign The Electrons To The Mo Provided In The Online Submission B Homeworklib
J. 590, 1131 (2003)] for dielectronic recombination (DR) of Mg2+. It is found that the choice of orbital description can lead to discrepancies by as much as a factor of 2 between total peak DR rate coefficients resulting from otherwise-identical computations. These unexpected differences are attributed to the large sensitivity to bound-orbital ...
Journal of Molecular Structure (Theochem),179 (1988) 145-152 145 Elsevier Science Publishers B.V., Amsterdam - Printed in The Netherlands A MOLECULAR ORBITAL STUDY OF A MODEL OF THE Mg2+ COORDINATION COMPLEX OF THE SELF SPLICING REACTION OF RIBOSOMAL RNA* MARY McCOURT ,b, MASAYUKI SHIBATAb, JAMES W. MCIVERa,b, Jr. and ROBERT REIN b,** 'Chemistry Department, State University of New York at ...
The molecular orbital diagram of BeCl2 will be drawn by combining atomic orbitals of beryllium atom and group orbitals of chlorine atom having similar energy and symmetry around a molecular axis. The 3s group orbitals of chlorine atom will remain non-bonding because their energy is very low as compared to the 2s and 2p atomic orbitals of ...
Get an easy, free answer to your question in Top Homework Answers. Predict whether or not a Mg2 molecule is stable. a. Mg2 is stable with a bond order of 2b. Mg2 is stable with a bond order of 0.5c. Mg2 is stable with a bond order of 1d. Mg2 is not stable Get an easy, free answer to your question in Top Homework Answers.
Place the following atoms/ions (Na+, Na, Mg2+) in order by increasing size. Mg2+ < Na+ < Na. What is the standard enthalpy of formation for CaCl2(s)? (Question 8 M3V3) ... Consider the following molecular orbital diagram for O2. Which molecular orbital below corresponds to the molecular orbital labeled with the number 3? (question 30 M3V3) III.
Potential curves of the A 1 Σ u+ - X 1 Σ g+ system of the Mg 2 molecule for J = 0 and J = 71. Reference. Figure 3. Potential curve for Mg 2 calculated within a basis of 56 contracted Gaussian type orbitals (the calculation of the correlation energy used 54 molecular orbitals since the 1σ g and 1σ u orbitals were frozen). Reference.

Fully Active Nitrogen Energetic Chains Mg2 N5 2n2 Mg2 N5 2n2 N Under Ambient Conditions Gao 2021 Advanced Theory And Simulations Wiley Online Library

The Energy Level Diagram Shown Here Can Be Continued To Higher Energies The Next Few Orbitals In Homeworklib
Beryllium Chemistry The Safe Way A Theoretical Evaluation Of Low Oxidation State Beryllium Compounds Dalton Transactions Rsc Publishing Doi 10 1039 C3dt50563d
Molecular Orbital Theory Chemistry Encyclopedia Structure Number Molecule Atom Bond Order Multiple Bonds

Mg2h2 New Insight On The Mg Mg Bonding And Spectroscopic Study The Journal Of Chemical Physics Vol 134 No 5
The Electronic Structure Of The Ground And Excited States Of Mg 2 And Mg2 The Journal Of Chemical Physics Vol 67 No 5

Construct The Molecular Orbital Diagram For H 2 And Then Identify The Bond Order Bond Order A 0 B 0 5 C 1 D 1 5 E 2 Image Study Com












0 Response to "38 mg2 molecular orbital diagram"
Post a Comment