37 c2 molecular orbital diagram
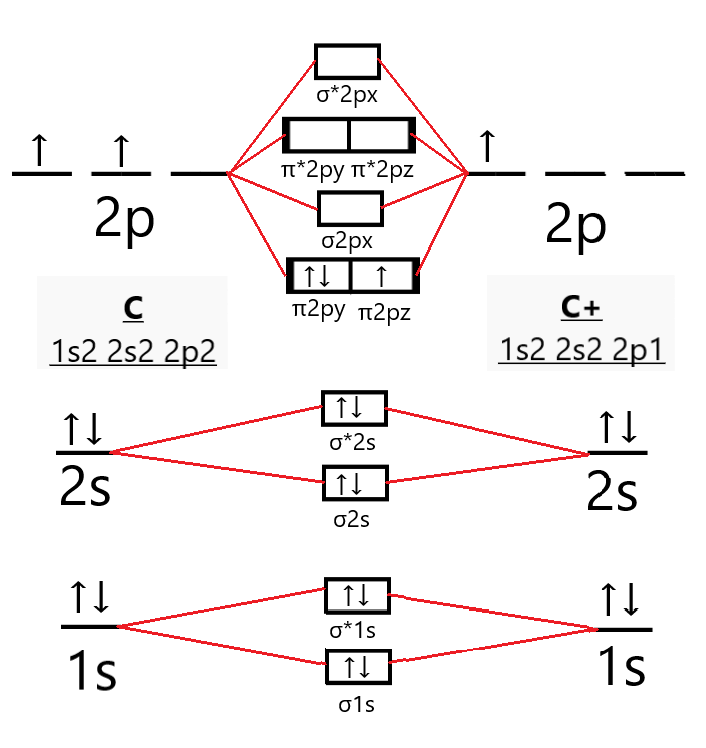
Construct the molecular orbital diagram for H+2 . A 1 s orbital from an H atom and a 1 s orbital from an H plus cation combine to form the molecular orbitals sigma 1 s and sigma 1 s star for the molecular H 2 plus. Sigma 1 s is lower in energy than the atomic orbitals and sigma 1 s star is higher in energy than the atomic orbitals. Identify the bond order. 0.5 Solution Start by determining the ... When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i...
C2 molecular orbital diagram. A mo is defined as the combination of atomic orbitals. As for bond orders it is 12e in bonding orbitals e in antibonding orbitals. The only orbitals that are important in our discussion of molecular orbitals are those formed when valence shell orbitals are combined.

C2 molecular orbital diagram
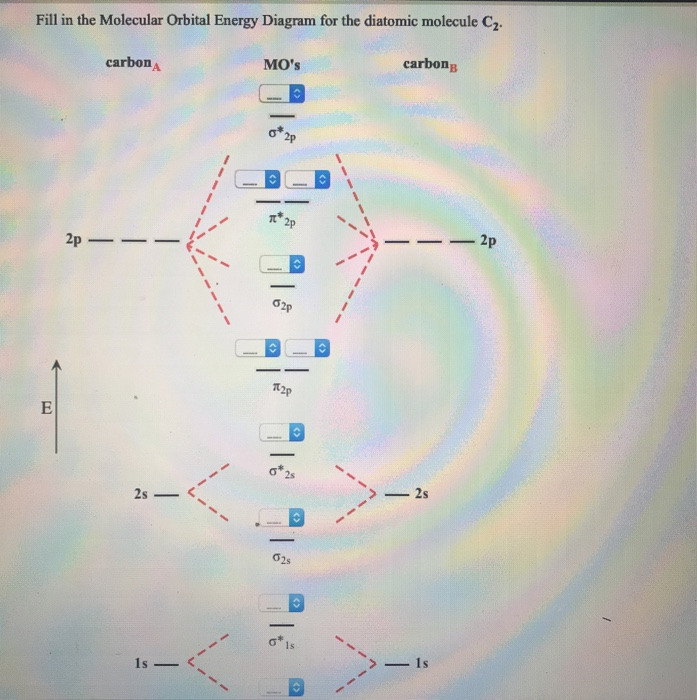
molecular orbitals in the diagram suggest a double bond. c. The σ2s, σ2s. *, σ2p, and σ2p ... The latter do not possess C2 rotation axes coincident to the.29 pages The diagram below shows the two $ 2p\pi $ orbitals, let's say $ 2p\pi x $ and $ 2p\pi y $ , are the highest energy occupied molecular orbitals. The lowest energy unoccupied molecular orbital is $ 2p\sigma $ , so that is where extra electrons will be added. C2 molecular orbital diagram. A diatomic molecular orbital diagram is used to understand the bonding of a diatomic molecule. Get 11 help now from expert chemistry tutors. Fill from the bottom up with 8 electrons total. They also give insight to the bond order of the molecule how many bonds are shared between the two atoms.
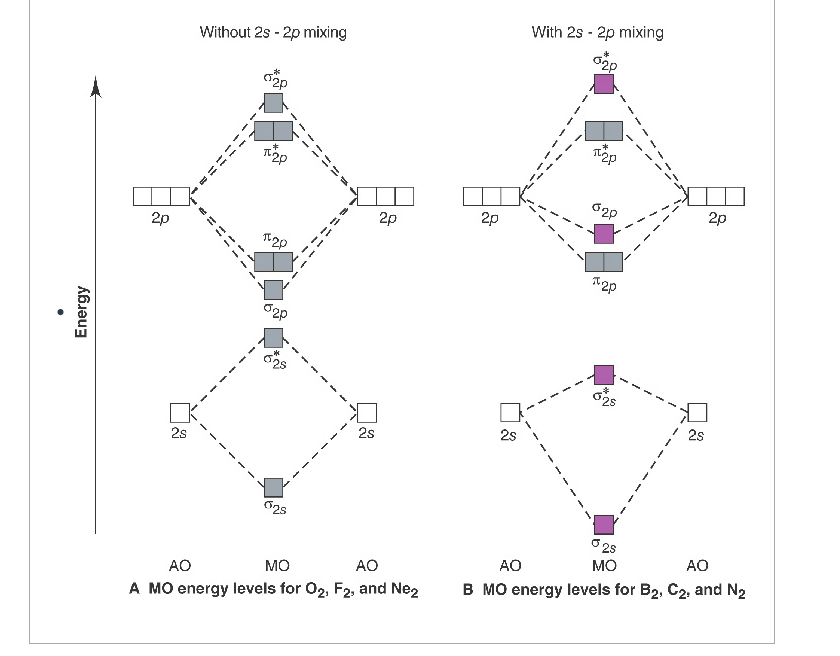
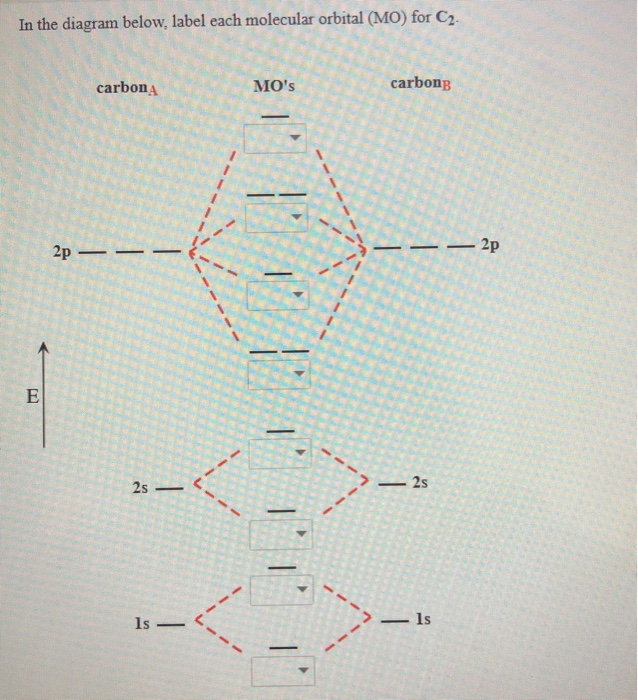
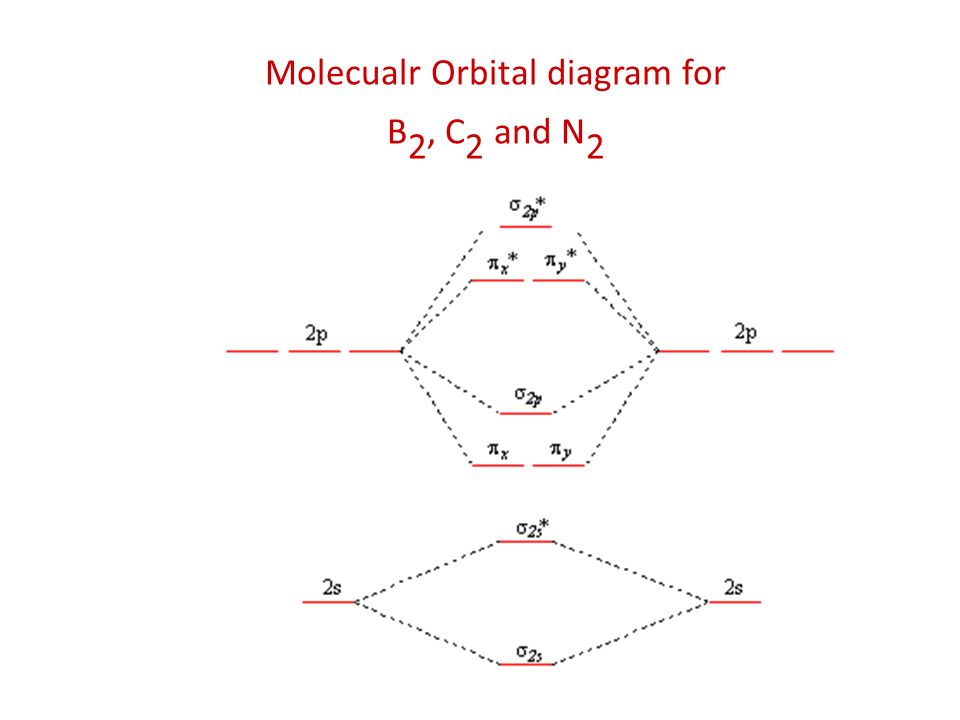
C2 molecular orbital diagram. C2 n2 o2 and f2 molecules diego troya. Molecular orbital diagram for carbon dimer c2. Molecular orbital diagram for the molecule oxygen o2. In the mo approach each carbon atom has four valence orbitals namely a 2s and three 2p. At the left of the table the energies are very close to each other and as a result the 2s and 2p orbitals mix with ... Molecular orbital diagram for c2. This video shows the mo diagrams of the c2 n2 o2 and f2 molecules. Molecular orbitals are formed combining similar atomic orbitals. Just because some chemical species shows integral value of bond order doesnt mean that it should exist. Molecular orbital diagram for the molecule oxygen o2. 15.08.2020 · The first molecular orbital results in the totally symmetric representation, working through all four operations E, C2, i, ?h will only result in 1's meaning there is no change, giving the Ag symmetry state. These molecular orbitals also represent different electronic states and can be arranged energetically. Putting the orbital that has the lowest energy, the orbital with the fewest nodes at ... 26.10.2021 · Li2 e. ! Σ g and σ u mos to be pushed apart in energy 8 atomic orbitals lead to 8 orbitals! For b2 c2 n2 o2 c2 f2 also c2 2- molecular orbital diagram diatomic Omonuclear molecules H2 c2. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular… Formation of N 2 molecule: Electronic Configuration, σ 1 s 2 . σ ∗ 1 s 2 σ 2 s 2 σ ...
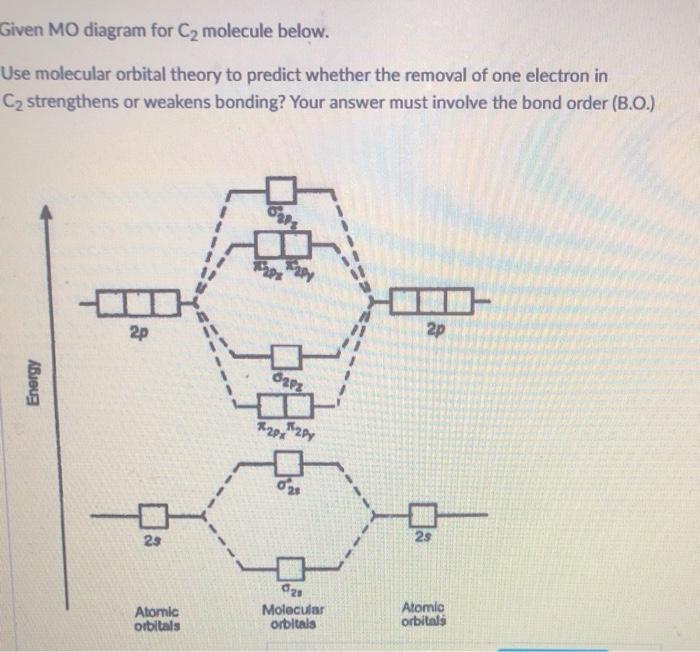
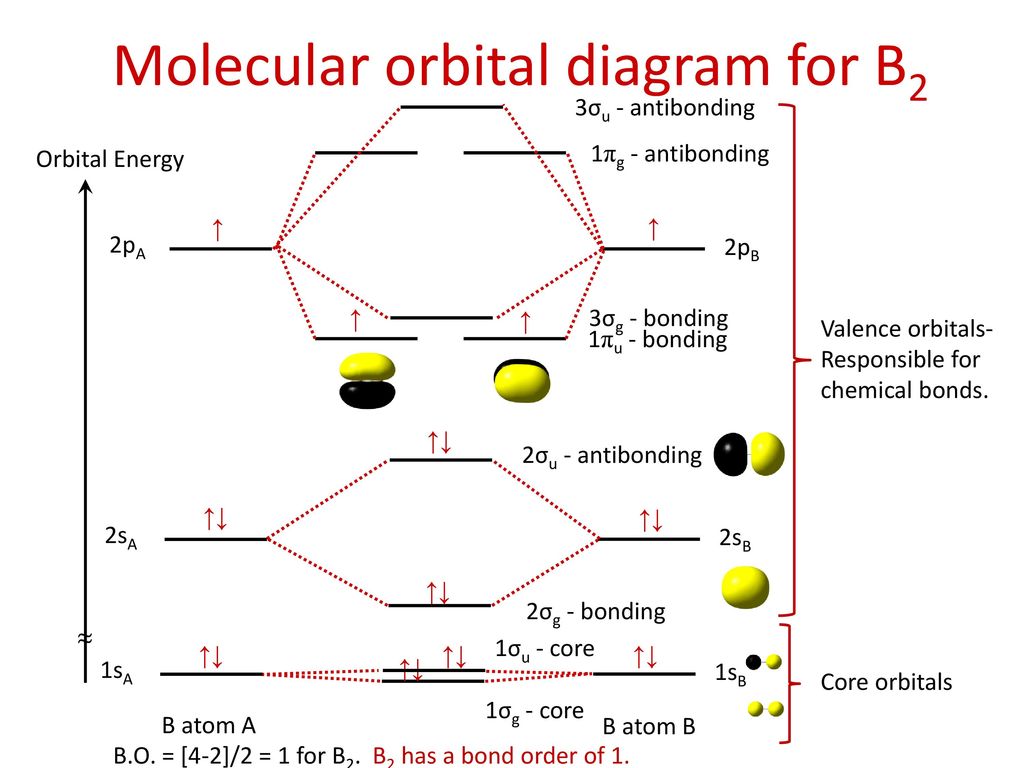
The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4. The electron configuration of the C− 2 ion will be. 12.09.2020 · Correlation diagram: A diagram which shows the relative energies of orbitals, configurations, valence bond structures, or states of reactants and products of a reaction, as a function of the molecular geometry, or another suitable parameter. An example involves the interpolation between the energies obtained for the united atoms and the values for the separated atom limits. 02.11.2021 · Molecular orbital energy diagram of b2. a. The next two would fill the 1 sigma e antibonding orbital. Individual atomic orbitals ao are arranged on the far left and far right of the diagram. As well, i filled in the sigma 2px and pi 2py orbits. Y: Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals ... 16.02.2017 · In the last post, we showed how to build a molecular orbital (MO) diagram for a typical C-C pi bond. We saw that: The number of molecular orbitals equalled the number of contributing atomic orbitals. The overlap of two atomic (p) orbitals gave rise to two molecular (pi, or π ) orbitals; The lowest-energy molecular orbital had all the phases in the contributing p-orbitals aligned the same way ...
Molecular Orbital Theory shows that there are two sets of paired electrons in a degenerate pi bonding set of orbitals. This gives a bond order of 2, meaning ...5 answers · 8 votes: C2 exists, but only above 3,642 °C (6,588 °F) i.e. in vapor state In chemistry, a conjugated system is a system of connected p orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as having alternating single and multiple bonds. Lone pairs, radicals or carbenium ions may be part of the system, which may be cyclic, acyclic, linear or mixed. Molecular orbital theory shows that it has two sets of paired electrons in a degenerate $\pi $- bonding set of orbitals. This gives a bond order of 2, which ... A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory ...
C2 2 molecular orbital diagram. Ion predictions with mo diagrams. Interact and form molecular orbitals. As for bond orders it is 12e in bonding orbitals e in antibonding orbitals doing this normally just c2 is 128 42. Molecular orbital diagram for carbon dimer c2. Give the molecular orbital configuration for the valence electrons in cec22.
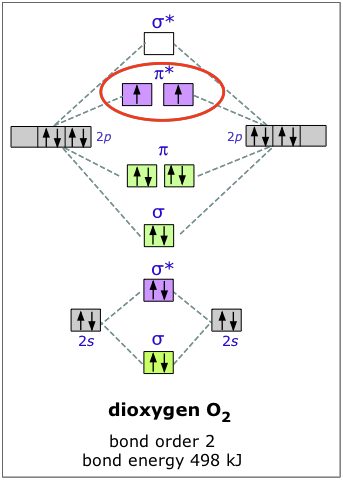
Molecular orbital diagram for carbon dimer c2. Molecular orbital diagram for n2 o2 c2 f2 also h2o. Fill from the bottom up with 8 electrons total. Mo diagrams can be used to deduce magnetic properties of a molecule and how they change with ionization. O2 2 Molecular Orbital Diagram Fabulous Electron Molecular Orbital.
Solved Predict The Magnetic Properties And Bond Orders Of A The Peroxide Ion O2 2 B The Acetylide Ion C2 2 Include A Sketch Of The Mo D Course Hero
Molecular orbital diagram for n2 o2 c2 f2 also h2o. Consider the h 2 molecule for example. Sp mixing causes the σ g and σ u mos to be pushed apart in energy. Molecular orbital diagram for n2 o2 c2 f2 also h2o this problem has been solved. A diatomic molecular orbital diagram is used to understand the bonding of a diatomic molecule.
Answer (1 of 7): UNDERSTANDABLE VERSION- See boy... If you are in class 11th then don't look for stuff like this. It hasn't been explained for a good reason. I will explain it to you in very crude terms but not its role in MOT as you wont be able to understand and I am saying this from experience...
08.08.2015 · The short answer is: we could not tell it using the primitive molecular orbital theory introduced in the general chemistry courses. In exact same way we could not tell why $\mathrm{\sigma_{2p_{z}}}$ MO becomes lower in energy than $\mathrm{\sigma_{2p_{z}}}$ MO to the left of $\ce{N2}$ and not to the left of, say, $\ce{C2}$. All this is simply because the primitive …
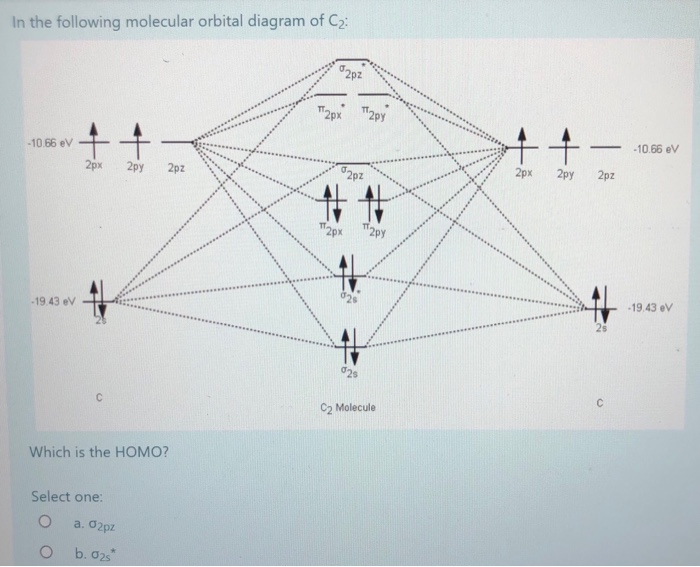
26.02.2018 · write molecular orbital configuration of c2+ predict magnetic behaviour and calculate its bond order. Asked by futureisbright051101 26th February 2018, 3:12 PM. Answered by Expert Answer: The molecular orbital diagram for C 2 molecule is : The electronic configuration of C 2 is K K (σ2s) 2 (σ * 2s) 2 n(2px) 2 n(2py) 2. The C 2 molecule is diamagnetic because all electrons are paired there ...

Molecular Orbital Theory Chemistry Libretexts In 2021 Chemistry Textbook Geometry Worksheets Electron Configuration
C2 molecular orbital diagram. A diatomic molecular orbital diagram is used to understand the bonding of a diatomic molecule. Get 11 help now from expert chemistry tutors. Fill from the bottom up with 8 electrons total. They also give insight to the bond order of the molecule how many bonds are shared between the two atoms.

Draw The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic N22 B22 Homeworklib
The diagram below shows the two $ 2p\pi $ orbitals, let's say $ 2p\pi x $ and $ 2p\pi y $ , are the highest energy occupied molecular orbitals. The lowest energy unoccupied molecular orbital is $ 2p\sigma $ , so that is where extra electrons will be added.
molecular orbitals in the diagram suggest a double bond. c. The σ2s, σ2s. *, σ2p, and σ2p ... The latter do not possess C2 rotation axes coincident to the.29 pages

Use The Molecular Orbital Energy Level Diagram To Show That N 2 Would Be Expected To Have A Triple Bond F 2 A Single Bond And Ne 2 No Bond

The Photoelectron Spectrum Of C2 Has Not Yet Been Measured Sketch A Predicted Spectrum Based On The Molecular Orbital Homeworklib
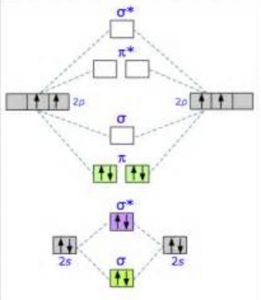
Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11

Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11

Is S P Mixing Referring To Hybridization Or Is It The Mixing Of One Atoms S Orbital With The Other S P Orbital Chemistry Stack Exchange
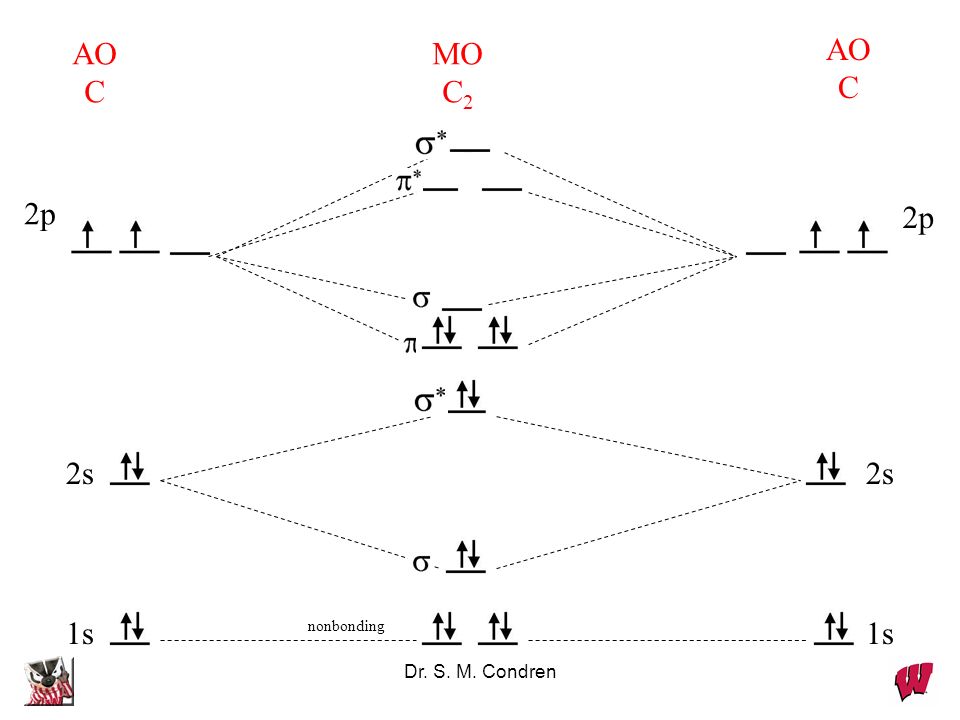
Chapter 10 Bonding And Molecular Structure Orbital Hybridization And Molecular Orbitals Dr S M Condren Ppt Video Online Download
Solved 5 Draw Complete Molecular Orbital Diagrams To Compare The Bonding In C2 F2 And Cf A What Is The Bond Order Of Each B Which Of The Thre Course Hero










0 Response to "37 c2 molecular orbital diagram"
Post a Comment