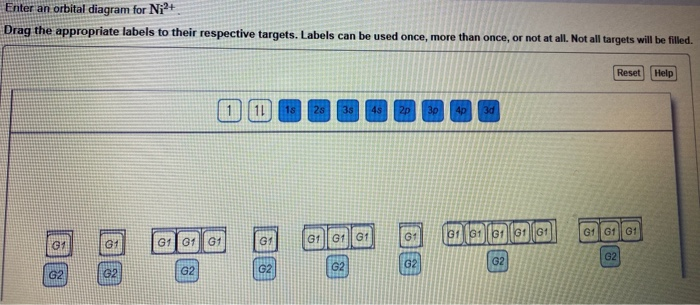
36 ni2+ orbital diagram
-Orbital diagrams are visual representations of electron configuration . Hund’s Rule •When electrons are filling orbitals of the same energy, they prefer to enter empty orbitals first. These electrons all have the same spin •A diagram of nitrogen is shown below (7 total There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start...
Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V 86%(14). Write orbital diagrams for each of these ions. A. V^5+ B. Cr^3+ C. Ni^2+ D. Fe^3+ E. Determine if the following ions are diamagnetic or paramagnetic.
Ni2+ orbital diagram
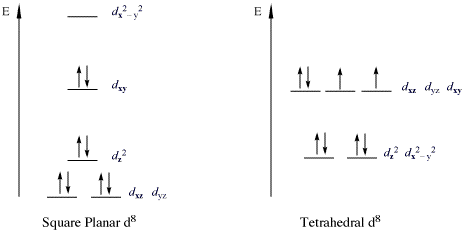
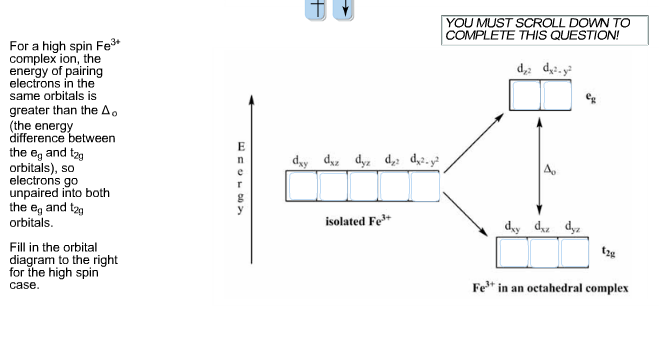
Ni2+ Draw the d-orbital splitting diagrams for the octahedral complex ions of each of the following. a. Zn2+ b. Co2+ (high and low spin) c. Ti3+ the FT ligand. Why isn't it [Ar] 4s2 3d5 (considering the fact that half filled orbitals confer If you have a gas phase Co(2+) ion, it would likely reorganize such. ... b. Cr3+ c. Ni2+ d. Fe3+. Answer. a. diamagnetic. (DIAGRAM NOT ... See the answer See the answer done loading. Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+. Determine if the ion is diamagnetic or paramagnetic. V5+,Cr3+,Ni2+,Fe3+. Expert Answer.
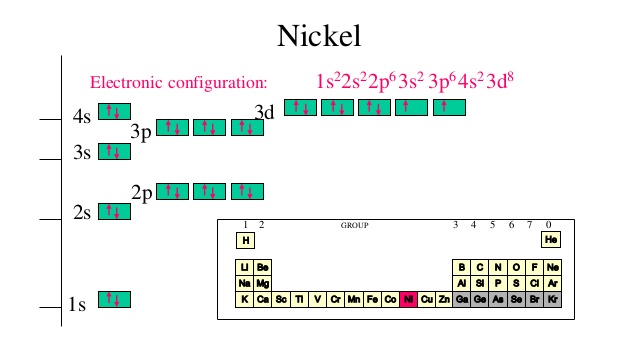
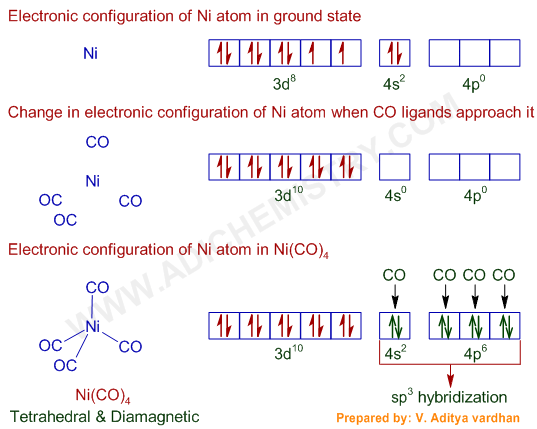
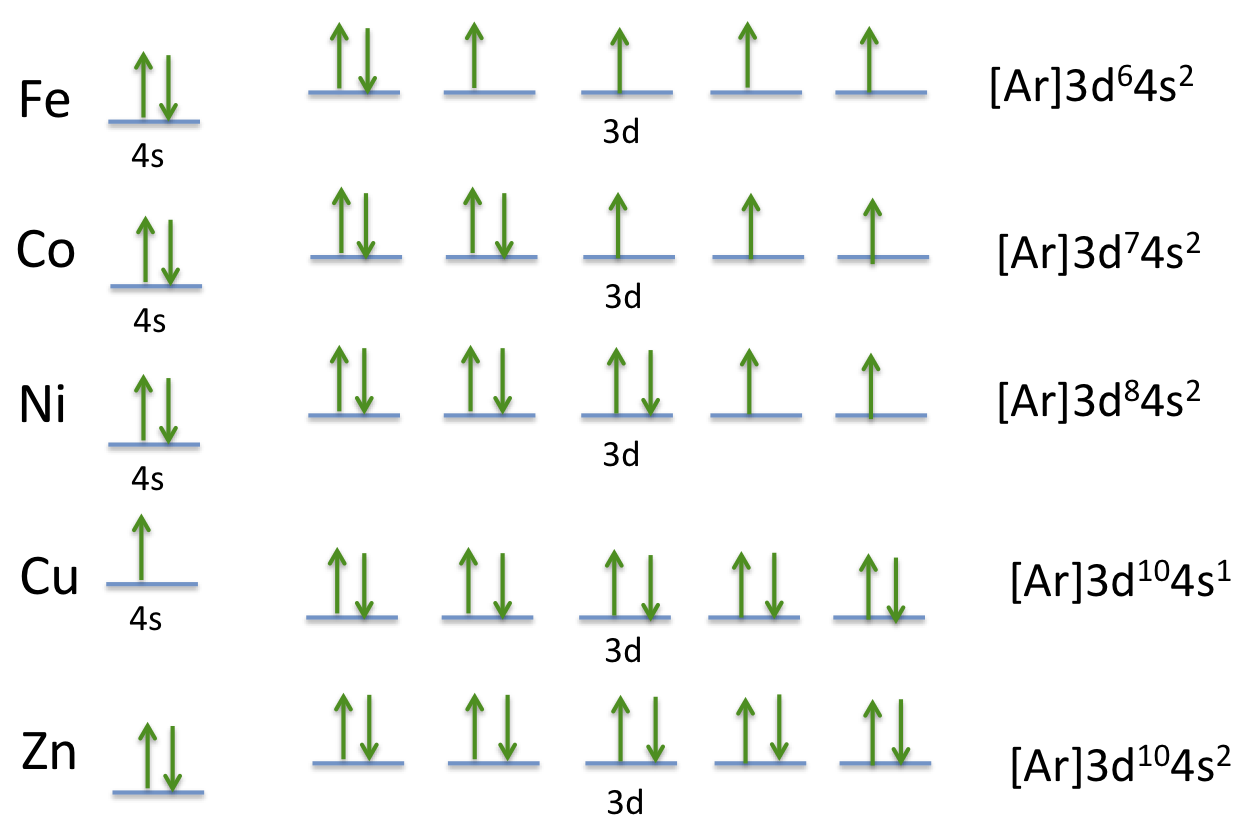
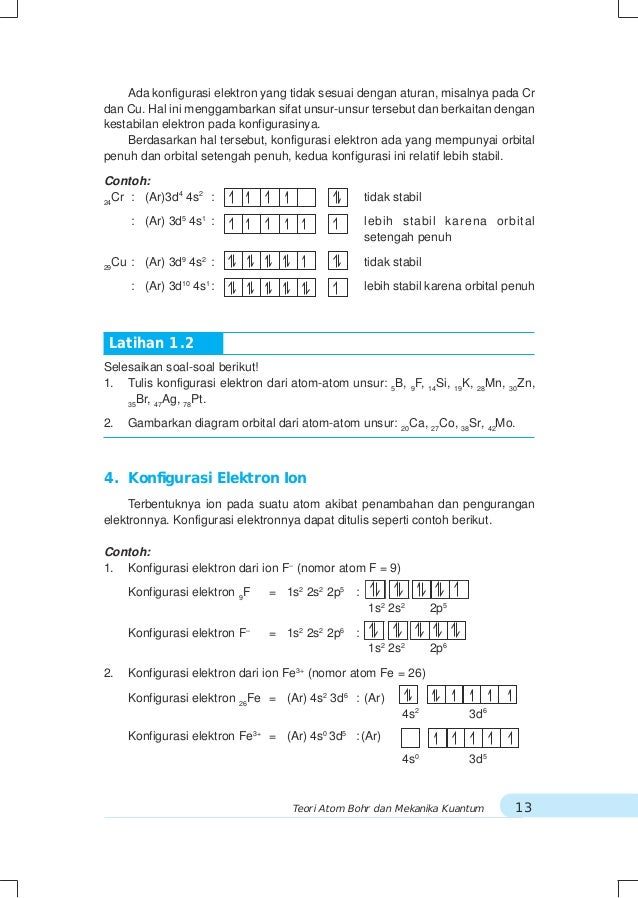
Ni2+ orbital diagram. Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. The 4s electrons a lower energy level that the 3d electrons because of the simpler electron path of the S orbital. So in the ground state the electrons ...2 answers · 1s22s22p63s23p64s23d6 in the ground state More correctly the electrons lost are the 4s2leaving ... In writing orbital diagrams, first, determine the electron configuration of the neutral atom and remove electrons accordingly. The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d8. In all of the cases, both up and down arrows are filled, with the exception of the 3d shell, where the last two are up ...
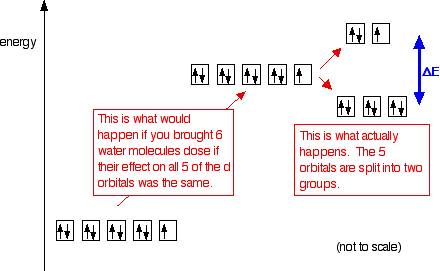
What is pseudotropism ? Question = Is ICl3 polar or nonpolar ? Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic. Recall that for: • diamagnetic: all of the electrons are paired Electromeric effect and mesomeric effect. Indicate if any overlap.Fe3+, Ni2+, Cu+, V3+, Mn4+ Q. a. Download scientific diagram | Schematic level diagrams of the Ir4+ 5d and Ni2+ 3d orbitals. The down-spin 3z2 − r2 electron mediates a FM coupling via a ... Answer (1 of 4): Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: 1. Aufbau Principle: Lowest energy levels fill first. 2. Pauli Exclusion Principle: Only 2 electrons per orbital, they must have opposite spin. 3. Hund’s Rule: Given sev... Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal –each electron occupies the lowest energy orbital available; German for “build up” •Electrons are notated with an arrow –Up arrow goes first then, down arrow –Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
Answer to: 1. Write orbital diagrams for each of these ions. *a. V5+ *b. Cr3+ *c. Ni2+ *d. Fe3+ 2. Determine if the ion is diamagnetic or...1 answer · Top answer: 1. The orbital diagrams are shown in the figures below. 2. V5+V5+ has all the electrons paired up so it is... See the answer See the answer done loading. Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+. Determine if the ion is diamagnetic or paramagnetic. V5+,Cr3+,Ni2+,Fe3+. Expert Answer. b. Cr3+ c. Ni2+ d. Fe3+. Answer. a. diamagnetic. (DIAGRAM NOT ... Ni2+ Draw the d-orbital splitting diagrams for the octahedral complex ions of each of the following. a. Zn2+ b. Co2+ (high and low spin) c. Ti3+ the FT ligand. Why isn't it [Ar] 4s2 3d5 (considering the fact that half filled orbitals confer If you have a gas phase Co(2+) ion, it would likely reorganize such. ...

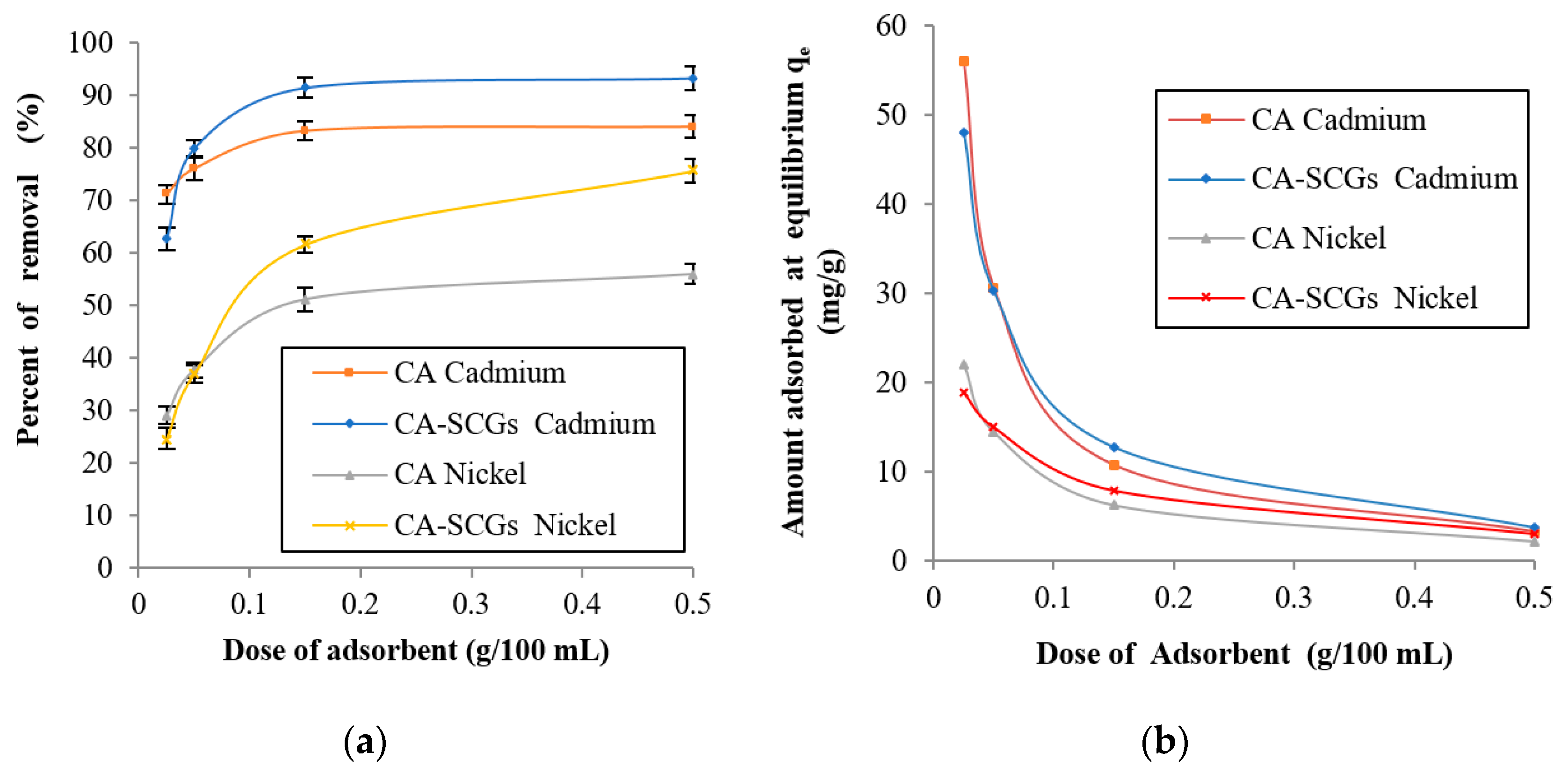
Applied Sciences Free Full Text Adsorption Of Ni2 And Cd2 From Water By Calcium Alginate Spent Coffee Grounds Composite Beads Html
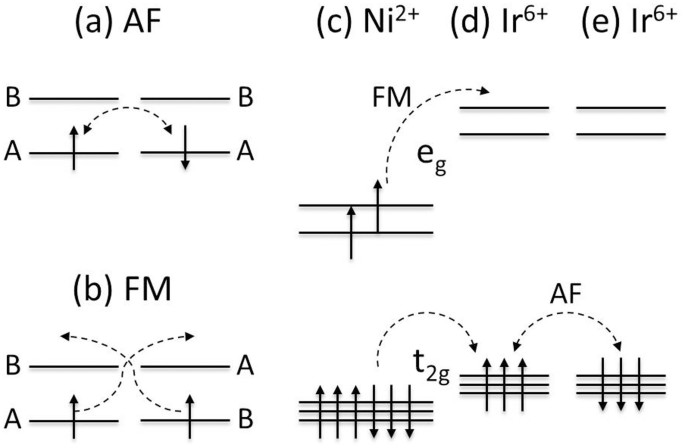
Long Range Magnetic Interaction And Frustration In Double Perovskites Sr2niiro6 And Sr2zniro6 Scientific Reports

Please Please Help The Orbital Diagram Below Is Written Incorrectly Why A The Number Of Electrons Brainly Com
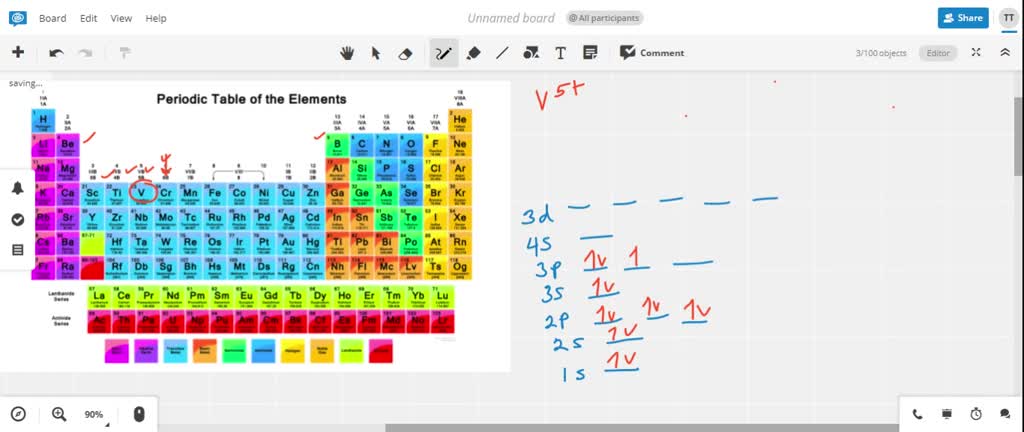
Solved Write Orbital Diagrams For Each Ion And Determine If The Ion Is Diamagnetic Or Paramagnetic A V5 B Cr3 C Ni2 D Fe3
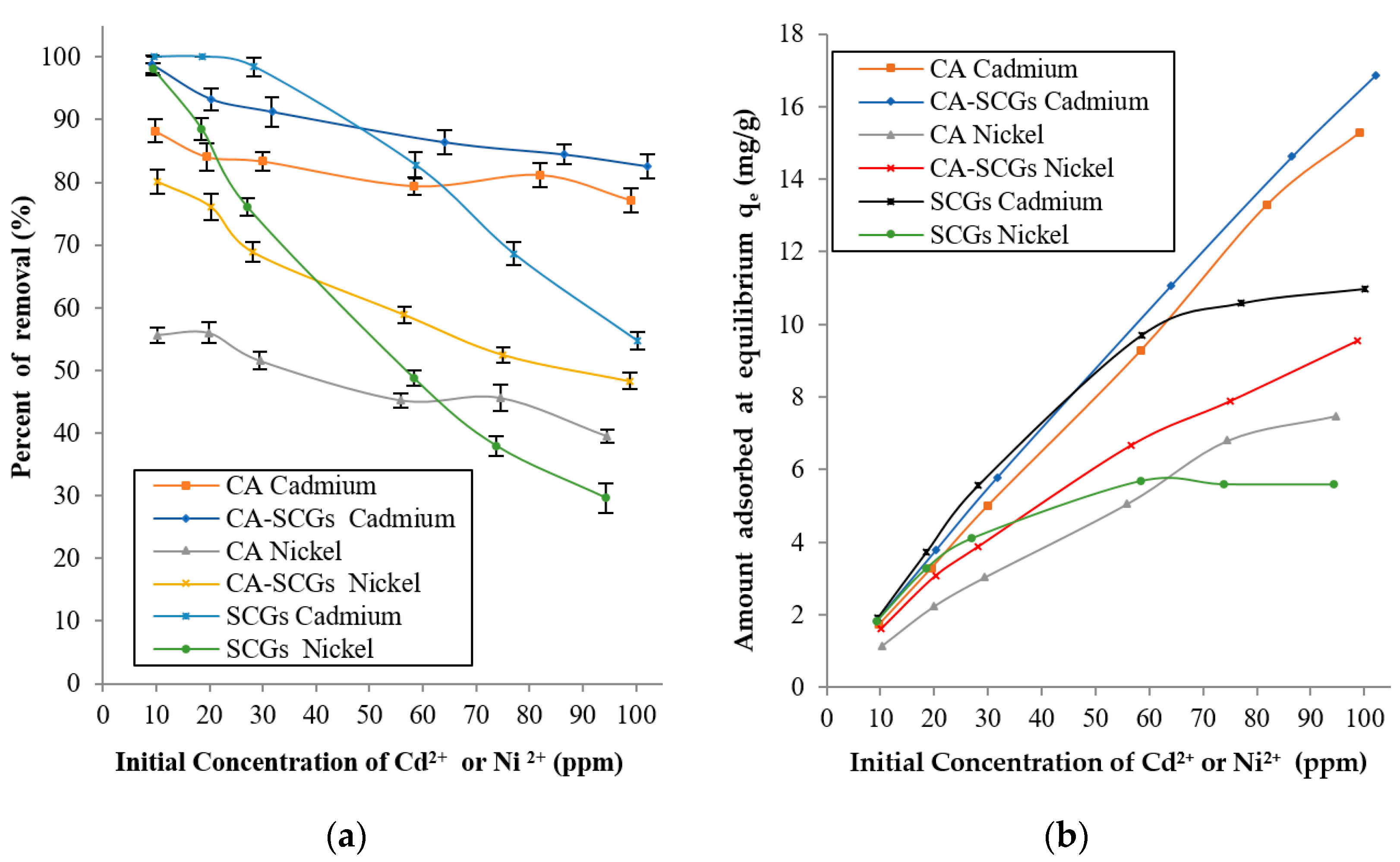
Applied Sciences Free Full Text Adsorption Of Ni2 And Cd2 From Water By Calcium Alginate Spent Coffee Grounds Composite Beads Html
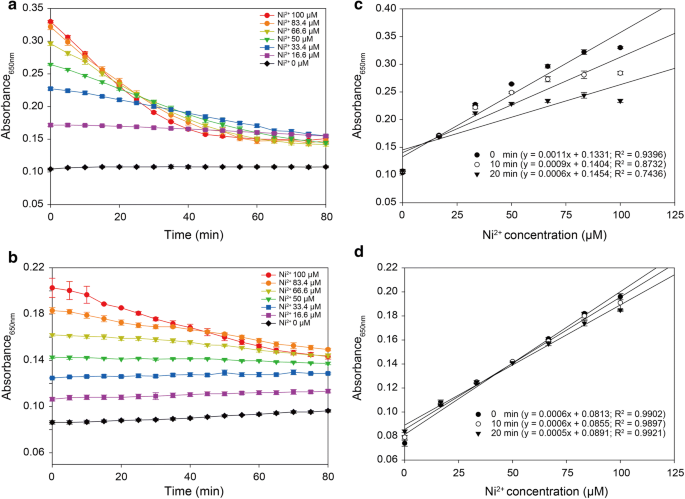
A Colorimetric Assay To Rapidly Determine The Activities Of Lytic Polysaccharide Monooxygenases Biotechnology For Biofuels Full Text

Outer Orbital Complexes Definition Octahedral Complex By Kakali Ghosh Teacher Blogger M Sc Chemistry Medium

State Yes No Whether Each Of The Following Orbital Diagrams Conforms To The Rules Governing Brainly Com

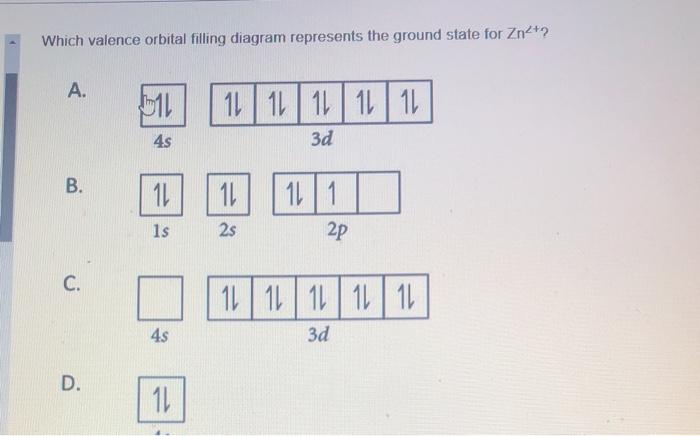
6 Give The Electron Configuration Use Inert Gas Symbol For Core Electrons And Orbital Diagram For Valence Electrons Homeworklib













0 Response to "36 ni2+ orbital diagram"
Post a Comment