35 orbital diagram for argon
Orbital diagram. Tags: Question 12. SURVEY. 300 seconds. Q. The electron configuration of an atom is 1s 2 2s 2 2p 6. The number of electrons in the atom is. answer choices. Answer (1 of 7): UNDERSTANDABLE VERSION- See boy... If you are in class 11th then don't look for stuff like this. It hasn't been explained for a good reason. I will explain it to you in very crude terms but not its role in MOT as you wont be able to understand and I am saying this from experience...
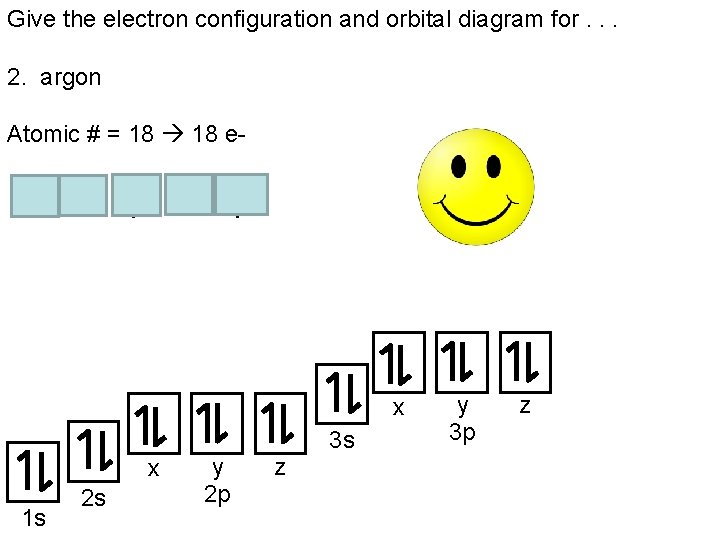
In order to write the Argon electron configuration we first need to know the number of electrons for the Ar atom (there are 18 electrons). When we write the configuration we'll put all 18 electrons in orbitals around the nucleus of the Argon atom. In writing the electron configuration for Argon the first two electrons will go in the 1s orbital.

Orbital diagram for argon
23 Feb 2012 — Convert from orbital representation diagrams to electron ... Now, let's draw the electron configuration for Argon and place the electron ... 2 Du 1 point Consider an argon atom in its ground state (lowest energy state): Look at the electron energy orbital diagram for argon shown below. Which orbital(s) shown in the diagram contain(s) electrons closest to the nucleus? Show your answer by clicking on the orbital(s) (1s, 2s, 2p, 3s, or 3p) in the image below. We thoroughly check each answer to a question to provide you with the most correct answers. Found a mistake? Let us know about it through the REPORT button at the bottom of the page. n atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other … Electron Configuration Practice Quiz Read More »
Orbital diagram for argon. This video shows how to create an orbital diagram of an atom from its electronic configuration Orbital diagram of Argon (Ar) 19: Orbital diagram of Potassium (K) 20: Orbital diagram of Calcium (Ca) 21: Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of ... Argon (Ar) has an atomic mass of 18. ... Electron Configuration, [Ne] 3s2 3p6. 1s2 2s2 2p6 3s2 3p6. Orbital Diagram ... Lewis Dot Diagram of Argon (Ar). Argon provides an inert atmosphere in which welded metals will not oxidise. Appearance. Argon is a colourless, odourless gas that is totally inert to other substances. Uses. Argon is often used when an inert atmosphere is needed. It is used in this way for the production of titanium and other reactive elements.
For example, sodium (Na), which has a single electron in its outer 3s orbital, can lose that electron to attain the electron configuration of neon. Chlorine, with seven valence electrons, can gain one electron to attain the configuration of argon. When two different elements have the same electron configuration, they are called isoelectronic. The sequence of orbitals for electron configuration can be seen from this diagram. The maximum number of electrons that can be filled in each orbital are: s = 2.1 answer · Top answer: Hey there! We have to draw the electron configuration for a neutron atom of argon.The electron configuration of an atom or molecule is the distribution ... The orbital diagrams below are of argon, nitrogen, and electron. image via galleryhip.com. image via chemistryland.com. image via chemwiki.ucdavis.edu. Unlike an s orbital, a p orbital points in a particular direction – the one drawn points up and down the page. At any one energy level it is possible to have three absolutely equivalent p ... 14.11.2021 · Molecular Orbital (MO) Diagram In VBT, also known as Valence Bond Theory, we consider the fact that atomic orbitals ( AOs ) from the same individual atom can come together to form fusion into hybridized orbitals which overlap with hybridized orbitals formed by the combination of AOs from other individual atoms inside the molecule.
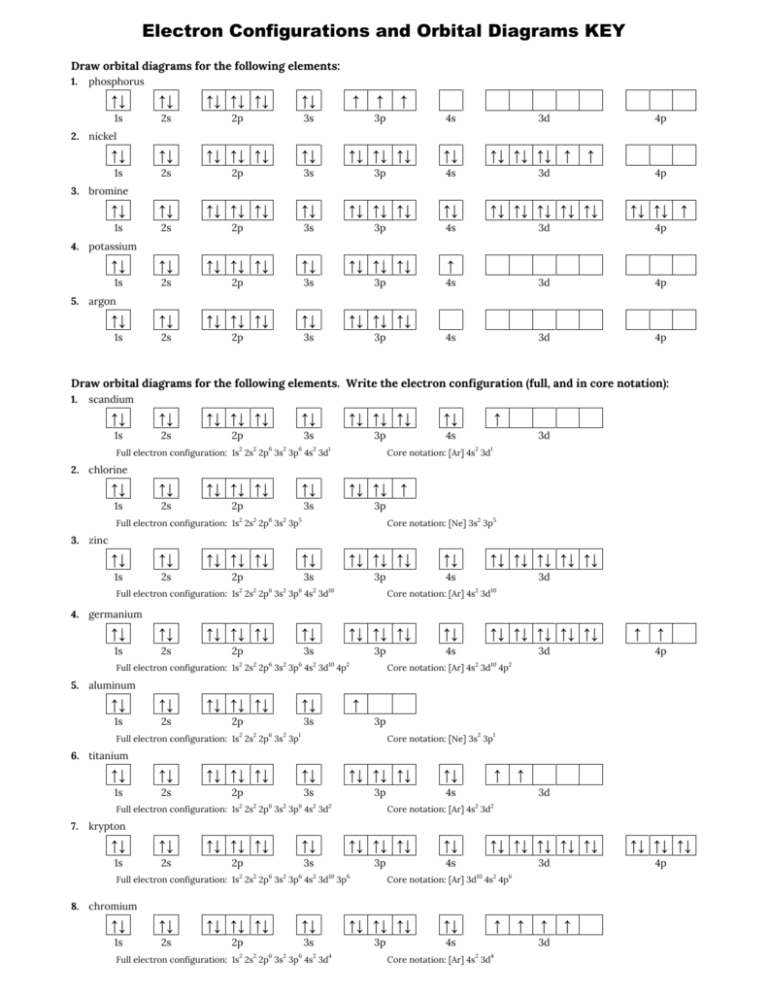
We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. We start with a single hydrogen atom (atomic number 1), which consists of one ... 15.08.2020 · Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially-filled valence shells and gain or lose electrons to achieve a stable electron ... The atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. If the atom is neutral, it will have the same number of negatively charged electrons. Its electron configuration is "1s"^2"2s"^2"2p"^2". The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with ... Below is the electronic diagram of the Argon atom. Orbital diagram of the Argon atom. Distribution of electrons over energy levels in the Ar atom
24 Jan 2021 — Argon's electron configuration for the first two electrons goes in the 1s orbital. 1s only hold two electrons and the next 2 electrons for Argon ...
Each box represents an orbital. Each orbital can hold up to two electrons. Ar. Question: Part A - Write the electron configuration for the Argon atom. Ch6: Photoelectron Spectroscopy. Seg1 Orbitals and Multi-Electron Atoms 114-OL- Course The electron energy-level diagram below shows the relative energies of orbitals for any multi- electron atom.
The orbital doagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-7.

Lewis Structure Nitrate Polyatomic Ion Molecular Orbital Diagram Potassium Nitrite Angle Text Png Pngegg
We thoroughly check each answer to a question to provide you with the most correct answers. Found a mistake? Let us know about it through the REPORT button at the bottom of the page. n atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other … Electron Configuration Practice Quiz Read More »
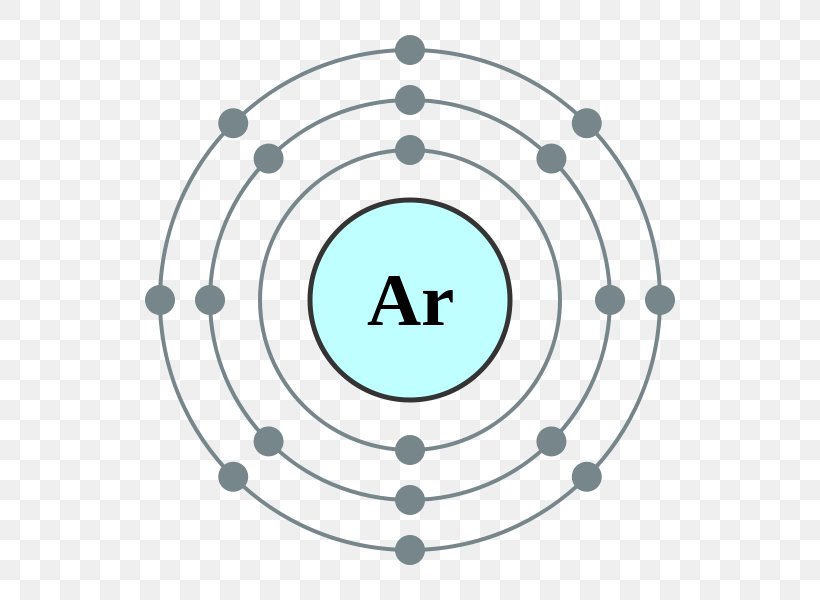
Electron Configuration Argon Atom Electron Shell Png 600x600px Electron Configuration Area Argon Atom Atomic Number Download
2 Du 1 point Consider an argon atom in its ground state (lowest energy state): Look at the electron energy orbital diagram for argon shown below. Which orbital(s) shown in the diagram contain(s) electrons closest to the nucleus? Show your answer by clicking on the orbital(s) (1s, 2s, 2p, 3s, or 3p) in the image below.
23 Feb 2012 — Convert from orbital representation diagrams to electron ... Now, let's draw the electron configuration for Argon and place the electron ...
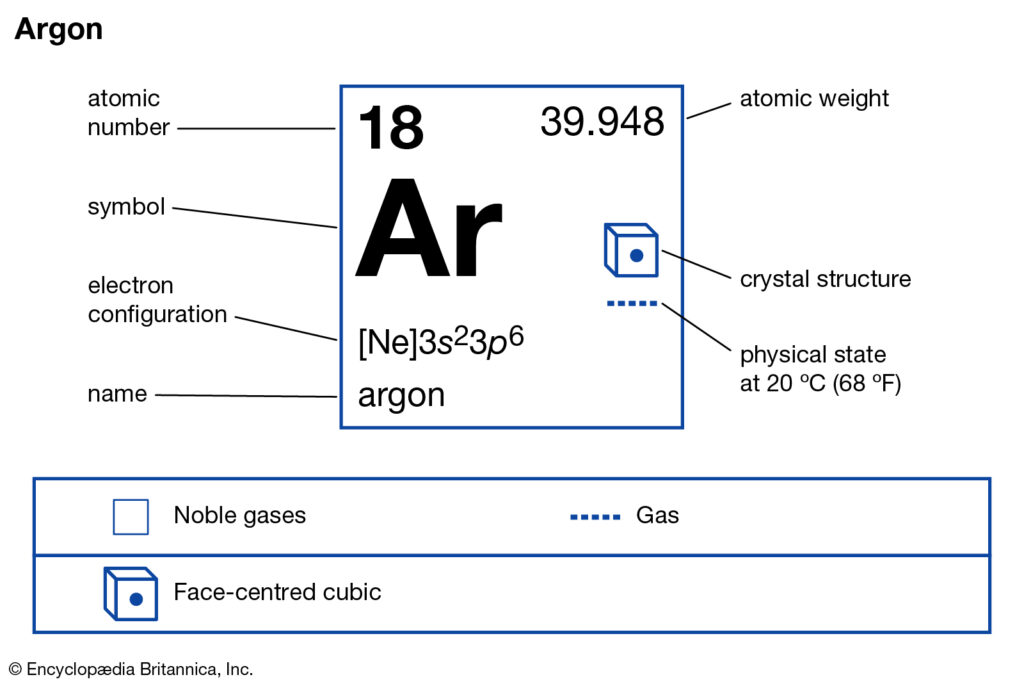
How Many Valence Electrons Does Argon Have Archives Dynamic Periodic Table Of Elements And Chemistry
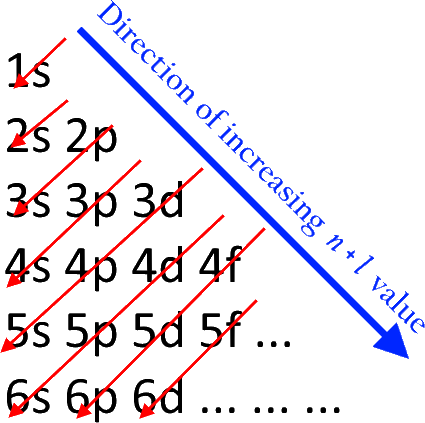
How Would You Identify The Period Block And Group Of The Element With The Electron Configuration Ar 3d 7 4s 2 Socratic
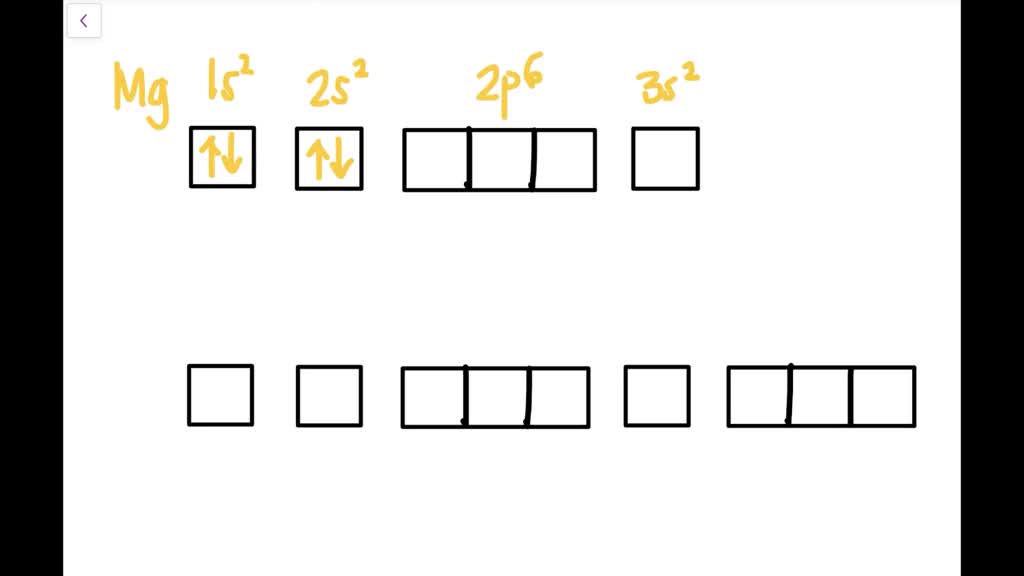
Solved Write The Electron Configurations For Mathrm Mg And Mathrm Ar Using Both Spdf Notation And Orbital Box Diagrams Describe The Relationship Of The Atom S Electron Configuration To Its Position In The Periodic Table




















0 Response to "35 orbital diagram for argon"
Post a Comment